Automobile Electrification and Heat Exchange
Chemical cleaning and passivation improves battery cooling and reliability in new energy vehicles.

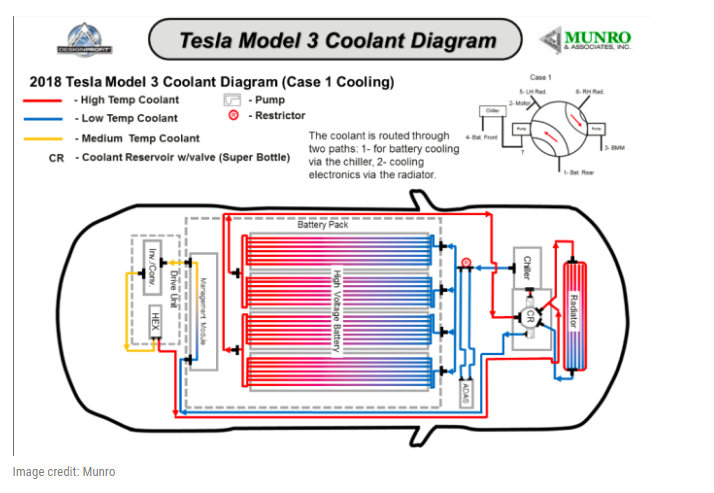
Tesla Model 3
Photo Credit: Tesla
Electric and other new energy vehicles (NEVs) are commanding an increasing proportion of the research and development and manufacturing investment in the automotive industry. With this new attention comes new requirements for Tier 1 Component Manufacturers. NEVs require a sophisticated cooling system of high-performance aluminum heat exchangers due to a different set of risks from the battery pack. At the same time, automotive OEMs are imposing new specifications for durability and longer service lifetimes.
In an internal combustion engine (ICE) vehicle, risk of failure mainly comes from the fuel tank, fuel plumbing, and engine. In the new energy vehicle, the highest risk comes from the possibility of cascading chemical reactions within overheated battery cells. Vehicle testing and field reports have found instances of cooling failure when heat exchanger channels are plugged into the radiator water-glycol loop, degrading system efficiency.
Featured Content
In vehicles with a large battery cell, temperature maintenance is achieved with a variety of techniques, including sophisticated temperature monitoring and energy usage software systems, which rely on functioning cooling hardware. Chief among these is the water-glycol cooling loop using the radiator, but a secondary method is use of the refrigerant loop used for cabin cooling. A new cooling plate is employed under the battery cells. The heat dissipating heat exchangers are similar in design to current ICE models and typically manufactured with the same production methods.
The predominant manufacturing process for automotive heat exchangers is controlled atmosphere brazing (CAB) with potassium aluminum fluoride flux, which acts as a deoxidizing agent in the brazing process, allowing formation of fillets which bind the components into a leak-free unit. Potassium fluoroaluminate (K-Al-F) residues are left within the heat exchangers and can slowly dissolve in water solutions, such as water-glycol mixtures. Residual flux interacts with coolant causing the coolant to gel. This gelling results in system clogging which in turn increases system operating pressure and temperatures, reduces system efficiency, and shortens system operating life. NEV systems, both electric and fuel cell, are more sensitive to this contamination so the focus on eliminating it has become heightened in recent years and will continue to be a key design consideration as engineers seek to maximize vehicle range, safety, and battery life.
Circle-Prosco Inc. has been working with leading automotive manufacturers and their Tier 1 suppliers to maximize the performance of aluminum heat exchangers since the U.S. market began to substitute aluminum for copper in the 1970s. In fact, providing antimicrobial conversion coatings to the aluminum heat exchanger industry is how the company started down the road of providing pretreatment products in the wide array of industries it serves today. Today, as it did in the 1970s, the automotive heat exchanger industry is undergoing something of a revolution. With expertise in the lab and in application, the company is working to meet the cooling challenges presented by NEVs head on.
To combat the possibility of cooling failure, Tier 1 heat exchanger suppliers are working to reduce the level of residual flux in two ways. First, they are re-evaluating their flux application process to reduce the amount of flux introduced to the system through more targeted use of potassium aluminum fluoride flux. Second, they are applying finishing industry knowledge and chemistry to the interior surface of heat exchangers.
As veterans of the finishing industry know, it takes a carefully balanced chemistry and process to clean stubborn residues off metal surfaces without damaging their structural integrity. Alkaline or acidic cleaners can remove flux and even dissolve aluminum if the flow, temperature, concentration, and dwell time are not dialed in. The first rule of removing residual flux is to do no harm to the heat exchanger!
Once the interior surfaces have been cleaned and prepared, it’s time for a conversion coating to passivate the surface of the metal. Zirconium-based pretreatments like Circle-Prosco’s Alcoat-5010 deliver exceptional corrosion resistance without sacrificing heat transfer. Non-chromate conversion coatings have supplanted chromate conversion coatings over the last 25 years. The Alcoat line of coatings are based on zirconium chemistry. Alcoat 5010 imparts a nanoscale coating that is covalently bonded and tenaciously adherent to aluminum surfaces. The nano-technology ensures that the heat exchanger channels remain unobstructed. Internal evaluation of surfaces and post cleaning burst pressure testing confirm the success of this process. The whole process can take anywhere from a few minutes to a few hours, depending on the condition of the parts at the start and the desired cleanliness when the part is complete. No matter the cleanliness specification, Circle-Prosco works with the OEM and Tier 1 supplier to optimize the chemistry, process, and quality controls system to ensure that the finished product meets or exceeds their customer’s requirements. Heat exchanger manufacturers can purchase chemistry to apply themselves with the support of Circle-Prosco or have the company clean and passivate the surface in-house.
As finishing professionals know, one of the first questions manufacturers ask about zirconium pretreatment is, “If it’s so thin, how will I know it’s there?” The same is true of heat exchanger manufacturers, but with another wrinkle — the coating is inside a closed heat exchanger unit. The industry has settled on several methods which correlate to the quantity of post-braze, post-passivation residual flux. Heat exchangers are filled with de-ionized water for a prescribed time period at a specified temperature. The resulting solution is analyzed for specifications, such as aluminum, with available chemical analysis techniques, for example, atomic absorption (AAS). As a faster and easier alternative, conductivity is employed. Conductivity is the detection of charge-carrying particles (ions) in a water solution. Solutions of low charged particle concentrations, (deionized water) have low conductivity, such as less than 10 microSiemens per square centimeter (μS/cm). Solutions with greater levels of dissolved solids will have greater conductivity. Circle-Prosco can run both tests in-house and works with customers to further optimize the passivation process or look for other solutions to improve incoming cleanliness before CAB brazing.
About the Author

Photo Credit: Circle-Prosco Inc.
Bill Morton
Bill Morton is Product Application Manager – Aluminum Coatings for Circle-Prosco Inc. Visit circleprosco.com.
RELATED CONTENT
-
VDA 19 and its Impact on European Manufacturing and Cleaning
The German Association of the Automotive Industry’s VDA Volume 19 is the first comprehensive standardization document for characterizing the cleanliness of products within the automotive industry’s quality chain.
-
Conversion Coatings: Phosphate vs. Zirconium
Both phosphate-based and zirconium coatings have their advantages, but zirconium is fast becoming the pretreatment of choice.
-
Stripping of Plated Finishes
The processes, chemicals and equipment, plus control and troubleshooting.


















