Pretreatment for Painting
Better adhesion, enhanced corrosion and blister resistance, and reduced coating-part interactions make pretreatment a must.
#basics
A high-quality conversion coating is essential for the durability of painted metal goods. The process of applying an inorganic conversion coating to a metallic surface involves removing any surface contaminants, then chemically converting the clean surface into a non-conductive, inorganic conversion coating. Conversion coatings increase the overall surface area and promote adhesion of the subsequently applied organic film. In addition, conversion coatings change the chemical nature of the surface, which increases corrosion resistance. It is these two functions, increasing surface area and changing the surface chemistry, that serve as a base for preparing the substrate material for paint finishes.
There are a number of driving forces in the pretreatment industry today with quality, cost and the environment being the most predominant. While these aren’t new issues, the pretreatment industry has responded to the needs of finishers by creating technology to address each of these requirements. In understanding the complete manufacturing process, including paint formulations, application equipment and regulatory impacts, it’s possible to address each driver simultaneously.
Featured Content
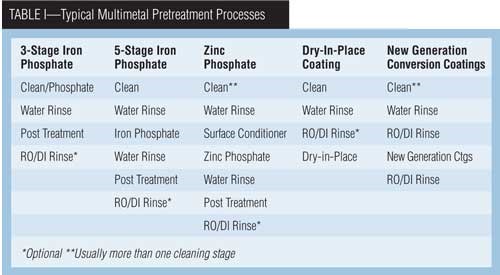
The conversion coating chemistries predominately used today are either zinc or iron phosphate. There is movement to replace these technologies with new types of phosphate-free or very-low-phosphate metal pretreatments. The new-generation technologies have been commercialized by many vendors over the past several years and are rapidly becoming industry standards. Regardless of the chemistry, conversion coatings are used to promote adhesion and improve corrosion resistance. Depending on the conversion coating and the desired performance, the conversion coating can be applied at a number of points in the process.
Cleaning
To increase the effectiveness of the finish, parts must be clean prior to coating. Aqueous cleaning, vapor degreasing or ultrasonic cleaning are typical cleaning processes, and of the three, aqueous cleaning makes up the majority. For parts that will subsequently be finished with organic coatings, surface pretreatment is required.

Zinc phosphating processes have been developed to provide exceptional painted part durability in corrosive environments. Typical industries using zinc phosphate processes include automotive and appliances.
Depending on the chemistry, iron phosphate systems can either be a cleaner coater, where the cleaning and coating take place in the same stage, or have a separate cleaning stage. Separate cleaning steps are essential for zinc phosphate systems and the new phosphate-free and low-phosphate conversion coatings. If the cleaner does not fulfill its purpose of removing unwanted soils from the substrate, subsequent processing steps will not produce a uniform conversion coating and therefore adequately protect the metal surface from corrosion.
Typical soils are either organic or inorganic. Rust preventative oils and lubricants, metal forming blends and rolling oils are examples of organic soils. Inorganic soils include mill or heat scale, metallic fines and laser scale.
Three types of cleaners are used in metal finishing: solvent cleaners, acid cleaners and alkaline cleaners. Using the proper cleaner for the application is critical, since the method of cleaning can affect coating characteristics such as coating weight and crystal structure as well as subsequent coating performance.
Solvent cleaners are usually used on small surface areas and offer limited ability to remove difficult oils. Solvents usage is diminishing in favor of more environmentally friendly options. Acid cleaners are chosen for removing inorganic soils such as surface oxides.
Alkaline cleaners deliver optimum results on organic soils. These cleaners are versatile enough to effectively clean the surface by lifting the soil up and dispersing it into the main cleaner bath, where it is held until it is removed mechanically using thermal oil separation, ultrafiltration or by overflowing the cleaner tank to drain off surface oils.
Rinsing
Proper rinsing is a critical, yet often-overlooked step in the pretreatment process. The water rinse process stops chemical reactions from taking place and removes unreacted chemicals from a part’s surface. Effective water rinsing also minimizes the migration of chemicals from one processing stage to the next. For effective rinsing, keeping the rinse water fresh reduces the amount of contamination present on the surface of the parts.
Since the key is controlling the amount of surface contamination on the part, if there is only a single rinse stage, a fresh water halo can be installed in between the chemical and water rinse stage rather than adding fresh water to the main tank. This allows the rinse tank to run at higher levels of contamination while the halo adds fresh water to the tank, but more importantly it floods the part and reduces the surface contamination. In the case of multiple rinse stages, they are counter-flowed and can effectively minimize water used in the rinse stages, requiring only a fraction of the water volume and reducing the amount of effluent produced. You can also reduce water consumption by optimizing your equipment design with proper racking of parts.
The Choices
Traditionally, the choices for a pretreatment process have been either an iron or zinc phosphate which provided the degree of performance necessary for the operation. Recently, there have been developments to replace this traditional technology with products that address ever-growing concerns with energy and water usage, environmental impact and the general operation of the process.
1) Iron Phosphate Pretreatment Systems. Iron phosphate systems, also known as alkali metal phosphates, are used for parts that require a durable finish but are not exposed to severely corrosive environments. These systems can involve two to six stages, with the shortest sequence being a cleaner-coater stage followed by a tap-water rinse. Short sequence systems are employed if performance requirements are low.
Parts that are more difficult to clean or have higher quality requirements call for a separate cleaning stage, appropriate rinse tanks, iron phosphate, post-treatment rinse and a DI rinse. A post-treatment rinse (chrome or non-chrome) results in improved corrosion performance over the phosphate alone.
Iron phosphates produce an amorphous conversion coating on steel that ranges in color from iridescent blue to gray, depending on operating conditions and product formulation. Mixed metals may be treated with modified formulas that typically contain fluoride.
Iron phosphate processes are much easier to operate and require fewer process stages than zinc phosphating. However, iron phosphates do not provide the degree of corrosion protection imparted by zinc phosphates.
2) Zinc Phosphate Pretreatment Systems. A zinc phosphate system varies from an iron system in two critical areas. First, it requires the use of a surface conditioner stage. Second, a zinc phosphate bath has additional metal ions in the solution which are incorporated into the coating along with the metal ions from the substrate being processed.
Surface Conditioning
Surface conditioning rinses are used in zinc phosphating to refine crystal morphology and control coating weight. State-of-the-art conditioners are liquid products that can be consistently applied using metering pumps.
The surface conditioning rinse takes place just before the zinc phosphate stage and is the only step in the process that is followed by another chemical stage, the zinc phosphate bath. The traditional surface conditioning chemistry is a colloidal suspension of a titanium salt. As these traditional baths age, they become less effective and must be dumped frequently or overflowed to maintain effectiveness.
Recently, zinc phosphate has been used to replace the titanium salt chemistry. This technology improves the refinement of the zinc phosphate coating, yet is not affected by the chemical make-up of the water or bath age.
Zinc Phosphate
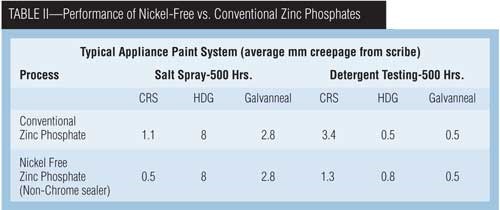
Zinc phosphating coatings provide exceptional painted part durability in corrosive environments and have the ability to coat mixed metals (steel, zinc-coated steel and aluminum). Several small developments have taken place over the past few years, such as decreasing the environmental impact, improved performance and ease of operation. New zinc phosphate systems operate at lower temperatures, in some cases are free of nitrites and nickel, and offer a reduction in sludge, and some products are internally accelerated. The objectives of the products were to increase quality, operate easily and, in the case of internally accelerated systems, eliminate the need for additional accelerators.
Depending on the metal mix in the system, additives are used to assist in the formation of the conversion coating on the substrate. For example, free fluoride added to the bath optimizes the conversion coating on aluminum and/or zinc. Adding calcium ions to the zinc phosphate bath produces a microcrystalline phosphate coating needed for rubber bonding. Depending on the final application and performance requirements, various other metal ions, organic acids, chelating agents and other chemicals can modify the overall characteristics of the zinc phosphate conversion coating.
Over the years, zinc phosphating systems have evolved from conventional systems containing high levels of zinc and nickel accelerated by sodium nitrite. An additional metal ion, manganese, was incorporated into the base chemistry to create the polycrystalline systems used today. Current polycrystalline systems can be either internally or externally accelerated, and in some processes, nickel was removed to create a nickel-free process.
New-Generation Conversion Coatings
New conversion coating technologies are being introduced that have four significant processing benefits. These coating processes are shorter, simpler and operate at lower temperatures than current zinc or iron phosphate processes. They perform well on all standard substrates of steel, zinc and aluminum. They significantly reduce the environmental impact, while their corrosion performance meets metal finishing specifications for painted metal substrates. All of these benefits provide significant cost savings to manufacturers willing to convert their existing processes.
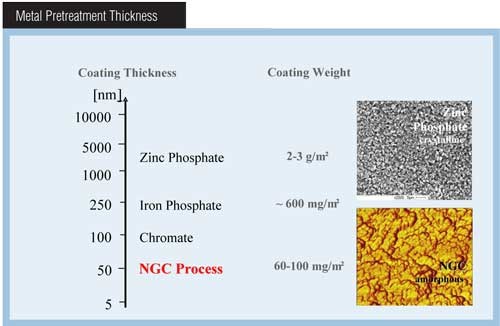
The new-generation conversion coating process is based on zirconium and additional propriety chemicals. When applied to a metal substrate, these chemicals react to form an amorphous zirconium oxide coating 20-80 nm thick that is significantly different from the iron phosphate and zinc phosphate coatings in use today. The new coating is thinner than traditional iron or zinc phosphate conversion coatings.
The new conversion coating process contains no zinc, nickel, manganese or phosphates; rather, it is based on zirconium containing chemicals. Zirconium is not regulated as a hazardous metal in North America or Europe. The new coating can be applied in fewer total stages than a zinc phosphate process and fewer chemical stages than both zinc and iron phosphate. In its simplest form, the process consists of five stages—two chemical stages and three water-rinse stages. The reduced number of stages will result in a 10- to 30-percent reduction in the overall plant footprint when converting from a standard zinc phosphate to the new-generation conversion coating process. A reduction in water usage also can be realized that is directly related to the reduction in process steps.
Post-Treatment
After a metal surface receives a conversion coating, the surface is water rinsed to remove unreacted chemicals, and a post-treatment may be applied. The post-treatment can increase corrosion and humidity resistance as compared with conversion coatings without final rinses. In the case of electrocoat applications, final deionized (DI) or reverse osmosis (RO) water rinse is required to minimize drag-in of high-conductivity water on the substrate surface from the post rinse. In these cases, it is imperative to have a reactive final rinse that maintains its properties after the DI or RO rinsing rather than a dry-in-place rinse.
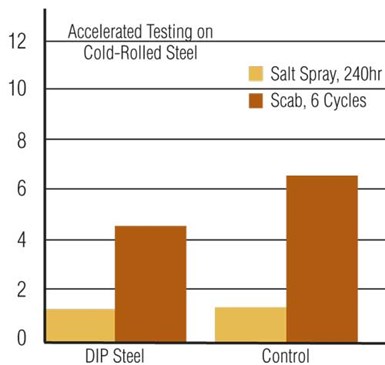
Polymeric dry-in-place seal versus standard iron phosphate.
Post-treatments historically have been based on chromic acid. With more stringent effluent guidelines, most finishers have converted to either trivalent-chrome or non-chrome post-treatments. The automotive industry has set standards to virtually eliminate the use of hexavalent chrome in vehicles produced after 2007. Recent advances in dry-in-place (DIP) polymeric post-treatments have shown excellent results when compared with standard non-chrome/DI rinsed systems.
Phosphate Coating Evaluation
There are three characteristics that define a conversion coating: the coating weight, the crystal size or morphology, and the chemical composition. When within the designed specifications for the application and material, all three characteristics contribute to ensuring the proper and expected adhesion and corrosion performance.
Coating Weight
Coating weight is defined as the amount of coating deposited within a specific surface area. Typically, coating weight is expressed in either grams per square meter (g/sq m) or milligrams per square foot (mg/sq ft). Each conversion coating technology is designed to deposit a specific coating weight on a substrate. Coating weight is an excellent indicator of whether the conversion coating bath is in proper chemical balance. If the coating weight is low and outside the specified range, something may be wrong within the process, and immediate attention is required.
Crystal Structure
The crystal structure of the conversion coating is measured through the use of a microscope, either an optical or, most commonly, a scanning electron microscope (SEM) at magnifications ranging from 100 to 1,000 times. In the case of new-generation coatings, this magnification is not sufficient, and other instruments such as an Atomic Force Microscope (AFM) or Field Emission SEM (FE-SEM) are necessary to achieve visible images of the coatings at 30,000 times and greater.
Regardless of the instrument required, the conversion coating is a combination of crystalline and microstructures chemically deposited on the metal surface. Instrumentation is used to determine the size, shape and uniformity of the coating. These instruments are excellent tools to examine any metal or coating imperfections that would go unnoticed with the naked eye. This visual examination of any non-uniform treated surface helps in the troubleshooting the pretreatment process. Through the use of the microscopes, the uniformity of the conversion coating can be monitored to ensure proper coverage and structure size. It is well-documented that structure size plays a very important role on paint adhesion.
Coating Composition
In addition to coating weight and crystal structure, the chemical composition of the coating plays a significant role in corrosion performance. Basically, corrosion is alkaline in nature, so the more alkaline resistance a coating delivers, the better the corrosion performance.
Chemical composition can be determined through a simple analysis in the laboratory using more advanced equipment, such as scanning electron microscopes with energy dispersive x-ray or x-ray diffraction. Use of this type of equipment is impractical on site, but can effectively evaluate performance in a laboratory setting. The chemical composition of the coating can help troubleshoot the process in applications where corrosion performance is below expectations.
Visual Inspection
Because the three coating characteristics take some time for evaluation, a simple visual inspection of the coating at the manufacturing site can look for problems. Phosphate coatings should be uniform in appearance whenever possible. Variations in color are normal on mixed metal sub-assemblies such as automobiles using various zinc-galvanized steel alloys.
Although color may vary, there should not be any visible shiny spots in the coating. Shiny areas indicate a condition known as inhibition. Inhibition is where the phosphate coating has not formed due to surface contamination.
Mapping is a widely used term today that describes various types of patterns that are visible on the conversion coating. These patterns are often most visible after the application of the paint film, making them extremely costly to repair. Mapping is normally caused by an uneven chemical reaction with the metal due to contamination such as oils, compounds, sealers or other materials left on the surface. The contaminants either react with the metal forming a permanent stain, or they are not removed by a chemical stage in process, i.e. poor cleaning, where the contamination is not removed in the cleaning stage and the next chemical stage must remove the surface contaminate. If this is the conversion chemical stage then the time needed to deposit the coating is compromised.
Patterns such as streaking may be a result of drying in drain vestibules, misaligned spray nozzles, or other air and solution flow imbalances within the phosphate system. In most cases, skilled operators can rapidly correct these patterns by realigning nozzles or adjusting pressures. In some instances, additional wetting harnesses are added to systems to address these problems. Some systems are incapable of correcting certain patterns due to original design flaws. Slight patterns are not normally detrimental to ultimate coating quality. The technologies that are available offer the metal finisher several options to choose from to meet a customer’s performance requirements. Currently, iron and zinc phosphate predominate as the products of choice when it comes to applying a conversion coating to a metal surface prior to painting. However, with the ever-changing business climate and the need to reduce costs, systems must become more environmentally friendly while reducing energy and water demands. With the development of the new-generation coatings, the landscape is changing, and the metal finishing industry has an opportunity to meet the cost and environmental constraints while meeting the performance expectations of its customers.
Johnson, E. "Pretreatment for Paint and Powder Coating," Products Finishing, 2002
Giles, T., "The Basics of Pretreatment," Electrocoat Conference Proceedings, March 1998
Goodreau, B., "The Basics of Metal Pretreatment," Electrocoat Conference Proceedings, May 2008
Fristad, W., "Phosphate Free Pretreatments for All Substrates," Coating Magazine Nov. 2008
RELATED CONTENT
-
Mitigating Inconsistencies From Liquid Coating Tank Changes
Mike Bonner of Saint Clair Systems discusses strategies for keeping liquid coatings homogenous and mitigating inconsistencies when changing process drums.
-
The Importance of Benchmarking Your Application Parameters
Having repeatability issues in your coating process? John Owed of Carlisle Fluid Technologies discusses the importance of benchmarking your application parameters in order to identify and quickly resolve problems.
-
Optimizing Ecoat With Zirconium Pretreatment
Darren Howard of Circle-Prosco discusses the ways zirconium pretreatments can provide a variety of benefits to your ecoat line.


















