What is Electrocoating?
E-coat can produce uniform finishes with excellent coverage and outstanding corrosion resistance.
#basics
Electrocoating is a process by which electrically charged particles are deposited out of a water suspension to coat a conductive part. During the electrocoat process, paint is applied to a part at a certain film thickness, which is regulated by the amount of voltage applied. The deposition is self-limiting and slows down as the applied coating electrically insulates the part. Electrocoat solids initially deposit in the areas closest to the counter electrode and, as these areas become insulated to current, solids are forced into more recessed bare metal areas to provide complete coverage. This phenomenon is known as throwpower and is a critical aspect of the electrocoat process.
Benefits of E-Coat
There are numerous benefits to electrocoating, including cost efficiencies, line productivity and environmental advantages. The cost efficiencies in electrocoat are higher transfer efficiency, precise film-build control, and low manpower requirements. Increased line productivity in electrocoat is due to faster line speeds, dense racking of parts, non-uniform line loading, and reduced human fatigue or error. The environmental advantages are no- or low-VOC and HAPs products, heavy metal-free products, reduced exposure of workers to hazardous materials, reduced fire hazards, and minimum waste discharge.
Featured Content
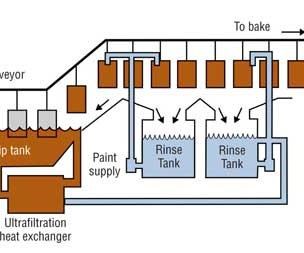
Four Steps of the Electrocoat Process
The electrocoat process can be divided into four distinct sections:
•Pretreatment
•E-coat bath and ancillary equipment
•Post rinses
•Cure oven
In a typical e-coat process, parts are first cleaned and pretreated with a phosphate conversion coating to prep the part for electrocoating. Parts are then dipped into a paint bath where direct current is applied between the parts and a “counter” electrode. Paint is attracted by the electric field to the part and is deposited on the part. Parts are removed from the bath, rinsed to reclaim undeposited paint solids, and then baked to cure the paint.
Seven Steps for Pretreatment in the E-Coat Process
Prior to paint film application, most metal surfaces receive pretreatment that usually involves a conversion coating.
The typical pretreatment process for e-coat consists of the following steps:
1) Cleaning (one or more stages)
2) Rinsing
3) Conditioning
4) Conversion coating
5) Rinsing
6) Post-treatment
7) Deionized water rinsing.
Phosphating processes can be separated into two types: iron phosphate and zinc phosphate. Iron phosphate has been the process of choice for applications where overall cost considerations override performance needs. Since iron phosphates are thinner coatings than zinc phosphates and only contain the metal ion of the substrate being processed, they provide reduced corrosion resistance compared with a zinc phosphate system. However, with environmental restrictions becoming increasingly tight with respect to heavy metals, an iron phosphate coating coupled with thorough post treatment may offer a viable alternative while still meeting required corrosion specifications. Zinc phosphates have become the preferred prepaint treatment in the metal finishing industry, especially with the use of electrocoat paint systems. The reason is that they provide better corrosion resistance and paint adhesion than iron phosphates under more demanding conditions.
Materials used in E-Coat
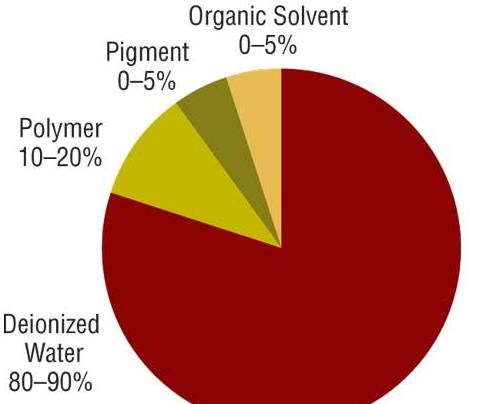
All coatings, including electrocoatings, are made from 1) polymeric resin or binder, 2) pigments, and 3) solvents and diluents. The resin (typically 10–20 percent) is the backbone of the final paint film and provides properties such as corrosion protection and ultraviolet durability. Pigments provide color, gloss and corrosion protection. Deionized water is the major component of an electrocoat bath, making up 80-90 percent of the bath. The deionized water acts as the carrier for paint solids, which consists of resins, pigments and small amounts of solvent. Solvents also help to ensure smooth film appearance and application.
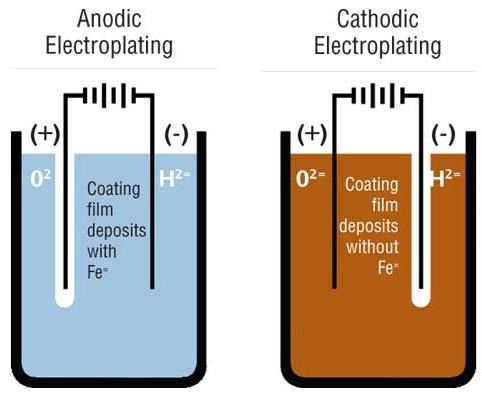
Electrocoat products are referred to as either anodic or cathodic, indicative of where coating deposition takes place. The process works on the principle of “opposites attract.”
Anodic electrocoating involves the use of negatively charged paint particles that are deposited onto positively charged metal substrates. During the anodic process, small amounts of metal ions migrate into the paint film, which limit the performance properties of these systems. These ions become trapped in the depositing paint film, and, due to their ability to interact with moisture, limit the corrosion performance of these films. Their main use is for products in interior or moderate exterior environments. Anodic coatings are economical systems and offer excellent color and gloss control.
Cathodic deposition, where positively charged paint particles are attracted to a negatively charged part, involves much less iron incorporation into the depositing film and consequently offers substantially improved corrosion resistance. Cathodic coatings are high-performance coatings with excellent corrosion resistance and can be formulated for exterior durability.
Types of Electrocoat
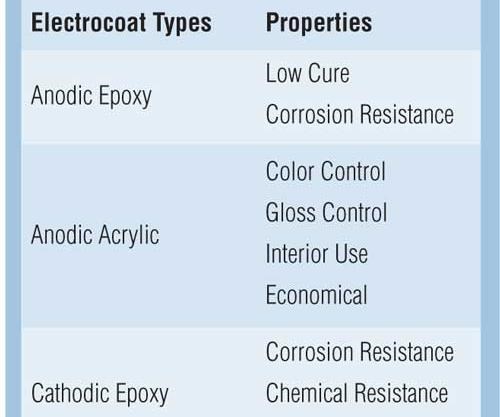
Acrylics, epoxies and hybrid formulations are offered to match the desired quality, performance, cost and environmental objectives that electrocoat paint manufacturers demand. Table I illustrates the properties for the four categories of electrocoats. Epoxy polymers are known for their corrosion and chemical resistance. Acrylic polymers are known for their ultraviolet durability and color control. Hybrids are a combination of epoxy and acrylic polymers that give a combination of properties.
Anodic epoxy electrocoatings are the most corrosion-resistant anodic electrocoat that can be cured at less than 200°F. The low cure attributes of anodic epoxies make these electrocoat products an excellent finish for castings, engines, and temperature-sensitive substrates or assemblies.
Anodic acrylic electrocoatings offer a single-coat application used for both interior and exterior environments and are available in numerous colors and glosses. In applications requiring a cost-effective way of applying a decorative or functional coating with good color control, anodic acrylic electrocoatings may offer the best value. These products are used as a one-coat finish on toolboxes, air diffusers, hangers, and other indoor or mild exterior environments.
Cathodic epoxy electrocoatings are the benchmark for corrosion resistance. Widely used in the automotive and automotive parts industries, they provide superior salt spray, humidity and cyclic corrosion resistance. However, the cathodic epoxy technologies generally require a topcoat to be protected from sunlight. Aromatic epoxy-type coatings are particularly prone to chalking and degradation by the UV components of sunlight.
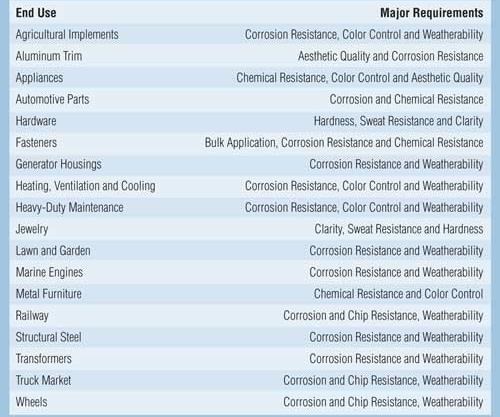
Cathodic acrylic electrocoatings are available in a wide range of glosses and colors to maximize exterior durability, gloss retention, color retention and corrosion protection. These products are used as a one-coat finish in the agricultural, lawn and garden, appliance, and air-conditioning industries. Cathodic acrylic electrocoatings are typically used in applications where both UV durability and corrosion protection on ferrous substrates (steel) are desired. The cathodic acrylics are also used in applications where light colors are desired. Some major markets that use electrocoating are listed in Table II.
Economics of Electrocoat
Electrocoat is normally the lowest-cost finishing application. The key word is “application.” Anytime one evaluates the cost to paint parts, he or she needs to look beyond just the material cost per square foot or the cost per gallon. Some of the major factors that enter into the equation for the application selection are:
•Part complexity. Parts come in all shapes and sizes. Electrocoat excels over other technologies in this category because all surfaces receive a consistent film thickness due to the electrical insulating effect of the electrocoat as it deposits onto the part.
•Production volume. As manufacturers increase production quantities above 2 million sq ft annually per shift, electrocoat becomes a more preferred application method. The dense rack loading achieved with electrocoat allows manufacturers to produce greater volumes of parts.
•Energy. All coatings operations require energy. Liquid and powder lines require daily rack cleaning and an additional dry-off oven after pretreatment. Powder lines normally require an environmental room to maintain air quality for consistent paint application to the part. Electrocoat has its share of energy requirements as well as requiring recirculating pumps, a chiller and a rectifier. The energy differences are not normally great when all factors are considered.
•Repair and maintenance. There is normally more mechanical equipment associated with electrocoat; however, the labor requirements to maintain a liquid or powder system are usually greater.
•Paint material. Electrocoat is usually the most cost effective when comparing applied paint materials. This is due to the high transfer efficiency (95–98 percent) and the self-limiting ability of the electrocoating process resulting in a differential of only 0.2 mils deposited paint film.
•Capital. In most cases, electrocoat lines require more capital dollars. But after other cost variables (film thickness, transfer efficiency and labor requirements) are taken into consideration, the electrocoat process produces the cheapest coating on an applied-cost-per-square-foot basis.
•Miscellaneous items. There will be additional overhead expenses such as insurance, building lease or mortgage, material handlers, shipping, etc., that the manufacturers needs to consider when determining the “final applied cost” for parts.
The choice of electrocoat over other coating technologies is usually driven by the lower total applied cost of electrocoat compared to technologies like liquid spray and powder coatings. The low total applied cost is achieved in part through more consistent deposition of paint film on parts (particularly complex geometries), high transfer efficiencies and the lower costs associated with labor.
Waste Water Treatment in the Electrocoat Process
Electrocoating is an extremely efficient process in which 95 percent or more of the paint components (resin, pigment and other additives) entering the paint tank will eventually be applied and cured on the product due to the recycling of paint through ultrafiltration. Except in rare cases of catastrophic tank contamination, the amount of paint solids requiring waste treatment on a regular operating basis is typically very small. Generally, discharge standards for wastewater become more stringent as they become more localized.
Federal standards can be found in the Code of Federal Register (CFR), Part 433— “Metal Finishing Paint Source Category.” This resource lists all registered substances that must be controlled at a national level. Local standards can vary greatly from municipality to municipality. In any case, they may be more demanding than the federal limits. Information on allowable contaminant levels may be obtained by contacting the local waste treatment facility. The permitting process can be quite complicated. It is suggested that a local environmental consultant be included in the permitting process to ensure that it is carried out completely and correctly.
Air Abatement in the Electrocoat Process
Electrocoat paint formulas have greatly reduced volatile organic compound (VOC) and hazardous air pollutant (HAP) content. Although solvent loading is greatly reduced, a source of VOC emissions will still exist as a result of the curing process. Ovens must be continuously exhausted to maintain the oven atmosphere below the lower explosion limit (LEL) and to prevent smoke buildup in the plant.
The resulting exhaust contains the released VOCs, smoke and odor from the cured paint, which may require treatment prior to release into the atmosphere. The selection of the proper abatement system is critical to the safe operation of the manufacturing facility, meeting air discharge standards, and minimizing odors for the surrounding area.
In any case, electrocoat paints have much lower VOC levels than solvent-based spray. And, just as some minute levels of contaminants may be legally discharged into sewer facilities, companies are allowed to exhaust some amount of VOCs into the atmosphere. This discharge is based on pounds released per year, and is set by federal and state Environmental Protection Agencies.
In addition, localities may establish further limits on emissions. Generally, requirements tend to be more stringent as the controlling body becomes more localized.
Recent Innovations in Electrocoat Applications
The use of electrocoat as both a primer and one-coat finish is increasing as formulators and finishers find more practical and economical ways to employ the technology. Companies are developing raw materials and formulations that improve performance (corrosion, UV durability, throw power, etc) in addition to phasing out the use of lead. Following are some examples of recent innovations in e-coat applications
•Home appliances. Home laundry appliances have traditionally required a high performance coating system using a two-coat application. A cationic epoxy primer was used for corrosion and detergent resistance. This required a liquid or powder topcoat for appearance consistency. Now a detergent-resistant cationic acrylic electrocoat supplies all of the requirements of the previous two-coat system. With this improvement, performance and appearance are at all-time-high levels. The advantages on cost, through automation and simplification, have kept this industry competitive in a price-conscious marketplace.
•Frames. The automotive industry standard for 20 years has been to coat the frames of cars and light trucks with hot wax, providing corrosion protection for one to three years. Hot wax required shielding from hot exhaust pipes and catalytic converters. The elimination of this shielding helped reduce weight and unwanted noise. New electrocoat products meet OEMs’ requirements for 10-year corrosion protection, demonstrating superior heat resistance and lower applied cost. Applications include truck and trailer frames, engine cradles, trailer hitches and tow hooks, suspension components, and underbody components.
•Decorative clears. The decorative-metal-finishing market has traditionally used spray and dip lacquer to achieve decorative clear finishes. New electrocoat systems designed to replace traditional methods provide higher transfer efficiency, reduced manual handling and fewer rejects. The products are formulated for application over a wide range of substrates, including plated gold, silver and brass, as well as copper, aluminum and steel base metals. Typical applications range from small components (cigarette lighters and jewelry) to larger household items (furniture and lighting fixtures).
•Decorative colors. Vibrant colors can now be achieved using decorative resins combined with specialty pigments. The unique pigment formulations allow for transparent colors which truly enhance the finished substrate. The colors can be used throughout the gloss range, high full through matte. The matte finish can resemble typical anodized finishes. The ability to replicate an anodized appearance makes electrocoat an attractive replacement for anodizing. Applications include automotive aftermarket, decorative, sporting equipment and lighting industries.
•Radiators. Radiators have historically been coated with a low-cost, low-performance, spray-applied black coating for cosmetic purposes. This poses several significant problems for heat exchangers. The lack of complete coverage leads to corrosion failure and core replacement. Cathodic epoxy electrocoat offered a promising solution to the corrosion problems encountered with heat exchangers. One of the advantages of electrocoat is its ability to provide complete surface coverage with unsurpassed film uniformity due to throwpower. Throwpower is the ability to throw paint into recessed areas. By being able to coat the entire core, corrosion resistance is greatly enhanced. Special epoxy electrocoat products, which have the ability to cover very sharp metal edges with a substantial film thickness of coating, further enhance corrosion performance over the fins in heat exchangers.
RELATED CONTENT
-
Measuring the Surface Area of Fasteners
How do you measure the surface area of a threaded fastener? How much coating would you put on it? How thick of a coating? What about non-threaded fasteners? The U.S. Department of Agriculture’s Forest Service, of all people, may have come up with the solution for those pondering how to coat sometimes-difficult small pieces using computer imaging and software to compute the area.
-
Coating Systems with the Best Long-Term Performance
The best protection against corrosion and UV exposure, says Axalta’s Mike Withers, is electrocoat and a super durable powder coating.
-
Conveyors and Paint Systems
Choosing the right conveyor system, coating technology, and ancillary equipment.