Electrolytic Versus Electroless Nickel Plating
Electroless and electrolytic nickel platings are effective at significantly improving a part’s strength and durability, but the differences between the two processes are important to distinguish.
#basics
Electrolytic and electroless nickel platings are processes that involve using special chemistries to deposit thin layers of nickel onto a metal or plastic substrate. Nickel is used because of its superior strength, durability, lubricity and corrosion resistance. These properties are essential for medical, semiconductor, military, and oil and gas industry applications where parts must withstand chemical exposure, heat and wear.
Both plating processes are effective at significantly improving a part’s strength and durability, but the differences between the two processes are important to distinguish.
Featured Content
Electrolytic nickel plating is categorized into two types: Type I and Type II. Type I dull nickel plating uses a sulfamate nickel solution that produces a dull, rough and porous coating which is highly ductile. Sulfamate nickel is commonly used for soldering or brazing. Type II bright nickel plating uses a similar solution with the addition of organic brighteners to produce a bright, chrome-like finish. This type of nickel electroplating is generally used as a topcoat to improve aesthetics and wear resistance.
Low, medium or high phosphorous chemical baths are used to provide a uniform final coating on machined parts. Successful deposition is dependent upon bath stability and a balanced ratio of chemicals in your solution.
- Low phosphorous (1-3% in deposit) baths produce a coating with hardness characteristics similar to bright nickel. These baths are very uncommon.
- Medium phosphorous (4-9% in deposit) baths are the most common and provide excellent corrosion protection. These are the most common baths used.
- High phosphorous (10-13% in deposit) baths can produce coatings that can even withstand exposure to highly corrosive materials. These baths are widely used by the semiconductor, food and beverage, and oil and gas industries.
When combined with a post-plate heat treatment, deposits of nickel-phosphorous achieve a hard, uniform coating that complete the manufacturing process at a component level.
Electrolytic Nickel Plating
Electrolytic nickel plating is a process involving the deposition of pure nickel using an electrolyte bath, conductive base and external electrical current. Parts, which vary in metallic composition, are thoroughly cleaned and submerged in the electrolytic bath that serves as the cathode. The nickel anodes, which reside in the bath continuously, are supplied with an electrical charge, thus releasing nickel ions that travel through the bath solution and attach to the surface of the cathode.
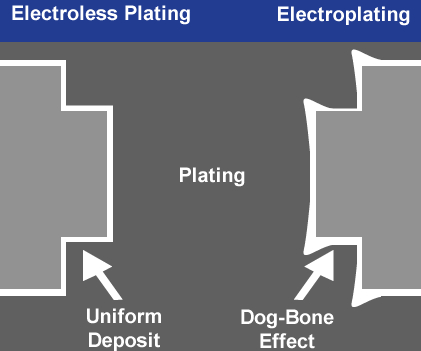
The coating thickness is controlled by the electrical current density and the amount of time the part is submerged in the bath. A possible disadvantage of this process is that applying an external electrical current to the bath creates a mixture of high-current and low-current areas on the part surface. This can result in nickel being unevenly deposited on the substrate, an effect known as dog boning. While it does not affect the ductility of the nickel plating, a lack of uniformity can impact dimensional tolerances.
Parts with varying thicknesses or intricacies are especially prone to dog boning, creating issues with uniformity. For these reasons, electrolytic nickel plating should be reserved for applications where ductility and purity of deposit take priority over coating uniformity, lubricity and corrosion resistance.
Advantages of Electrolytic Nickel Plating
- Pure nickel deposit
- Ductility high enough to meet or exceed AMS 2424
- Withstands post-plate firing temperatures of 1,000°C (1,832°F)
- Microhardness typically ranges below 300 HV
Electroless Nickel Plating
Electroless nickel plating is the deposition of a nickel-phosphorous alloy using an autocatalytic bath. The part is submerged and a series of chemical reactions take place which deposit metal ions onto the substrate without introducing an outside source of electrons.
This autocatalytic process used to be incredibly complex and, therefore, was not widely recognized or accepted. Over the past 20 years, however, electroless plating has proven to be a process that provides better corrosion and wear resistance than electrolytic nickel plating. It is also widely used as an undercoating that enhances the wear-resistant properties of silver, gold and copper coatings.
A crucial part of electroless nickel plating is managing pH, hypophosphate and nickel content to achieve the correct deposition rate for the alloy you are plating. With the right chemistry, electroless nickel plating creates a uniform, nonporous coating with excellent lubricity and corrosion resistance and also eliminates the issue of dog boning. Electroless nickel plating rarely requires additional post-plate modification.
Advantages of Electroless Nickel Plating
- Excellent lubricity
- High corrosion resistance
- Less likely to experience overplating
- No high-current density issues or dog boning
- No need for an external source of electrons
- Can be deposited on virtually any metal or alloy
- Uniform deposition, even on complex geometries
- Compatible with conductive and nonconductive materials
- Usually does not require additional post-plate modification
- As plated hardness of approximately 500 HV
- Post-plated hardness between 800 and 900 HV
Electroless and electrolytic nickel plating each have advantages, but are only effective when a company has proper quality control and surface preparation standards in place. If the wrong chemistry is used or parts are not left in the bath for the right amount of time, it can produce a poor finish, resulting in scrapped parts.
ENS Technology’s predecessor started operation in 1975, before today’s state-of-the-art nickel plating processes were a common practice. Over the years, we have developed our own chemistries to achieve the ideal coatings. Our team has over 300 combined years of experience in electroless and electrolytic nickel electroplating, and analyzes plating baths throughout the day to ensure proper chemistry. We can deposit nickel phosphorous with a 4-13% phosphorous content based on each company’s unique application and needs.
Visit enstechnology.com.
RELATED CONTENT
-
A Chromium Plating Overview
An overview of decorative and hard chromium electroplating processes.
-
Aluminum Surface Finishing Corrosion Causes and Troubleshooting
In this paper, a review of several process solutions, examining coolants, solvent cleaning, alkaline clean/etch and deoxidizing/desmutting, listing intended and unintended chemical reactions along with possible mechanisms that would favor corrosion formation.
-
Zinc Electroplating
Choosing the best process for your operation.