Gold and Silver Plating Basics
An overview of precious metal electroplating processes.
#basics
Gold and silver share a number of chemical and physical properties. Both are soft, malleable and ductile materials, with melting points of 1,065°C and 961°C, respectively. Both crystallize in face-centered cubic configurations and form stable alloys with each other in all proportions. Both resist most common acids, although silver is readily attacked by nitric acid, and gold by aqua regia. Both form complexes with alkali
cyanides.
Because of their relative rarity, both have been sought after since ancient times. Much of recorded history involves them, and they continue to be valued for decorative, monetary and usage-driven applications.
Featured Content
Gold Plating
Early applications of gold plating and its predecessors, fire gilding and leafing, were almost exclusively decorative. The rise of the electrical and electronics industries in the latter half of the 19th and throughout the 20th centuries created applications for gold based on its chemical inertness, low and stable contact resistance, conductivity, and resistance to arcing.
Because of its price, it has always been important to deposit gold only in those areas and at such thickness as the application actually requires. Thus, selective techniques for gold deposition were developed quite early on and continue to be improved.
Cyanide-Based Gold-Plating Systems
Most plating of gold and its alloys is carried out from solutions containing gold as a soluble cyanide complex. Gold reacts with alkali cyanides to form either monovalent (MAu(CN)2) or trivalent (MAu(CN)4) complexes, wherein M is an alkali metal or, in some cases, ammonium ion. Gold plating solutions, as originally configured, contained some excess of free alkali cyanide, which served as a portion of the electrolyte. This automatically established the solution pH in the range of about 10 to 12; and since cyanide is highly surface active and difficult to rinse, caused occasional staining problems as well.
Around 1950, it was recognized that monovalent alkali gold cyanides were stable in water at pH values as low as 3.2. Trivalent alkali gold cyanides are stable almost to pH zero. This realization allowed the development of purpose-designed gold plating electrolytes, free of excess cyanide and operating over a wide range of pH; containing, as necessary, various additives for brightening, hardening, grain refinement, depolarization and/or range extension.
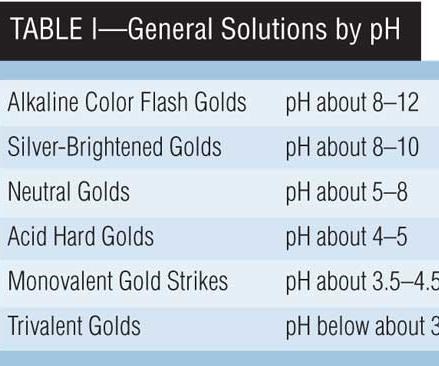
Over time, a number of general solution classes have evolved, some of which may be listed in order of decreasing pH, as found in Table I.
Alkaline Color-Flash Golds
Color-flash golds are formulated to produce thin coatings of a definite color as a final finish to work that has previously been prepared. Color-flash solutions typically contain quite small amounts (0.5-1.5 g/L) of gold. Potassium gold cyanide, KAu(CN)2, together with dipotassium or disodium phosphate, is the normal electrolyte containing a small amount (0–7.5 g/L) of free KCN, and additives such as KAg(CN)2, KCu(CN)2, and/or K2Ni(CN)4 in amounts sufficient to produce the desired color. The solutions are operated at temperatures of 50–70°C and plating is usually done at 4 to 6 V for 10 to 12 seconds.
Color-flash golds typically contain no brighteners, and since the deposits are very thin (0.025–0.075 μm) and highly alloyed, they are not abrasion-resistant. Good practice requires applying a layer of abrasion-resistant hard gold prior to the final color flash.
Silver-Brightened Golds
Silver was the first commercially successful brightening agent for gold. Early formulations of this system, as well as of similar formulations employing antimony or tin, made use of large concentrations of free cyanide (90-120 g/L), which caused staining problems and attacked printed circuit board laminates, then in use. These solutions have been
reformulated with electrolytes similar to those of the color-flash baths, based on phosphate and with the free cyanide reduced to 0-7.5 g/L. Deposits with silver content in the range of about 4 to 9 weight percent are particularly durable in sliding friction, and are still used for slidewire and rotary-switch applications.
Neutral Golds
Neutral golds comprise a very large class of electrolytes aimed at deposition of very pure, soft deposits for electrical and electronic applications. There is no free cyanide. Electrolytes are based on phosphates, phosphonates or the salts of various organic
acids. Most neutral gold solutions operate in the pH range of about 6 to 7, although it is common to raise pH somewhat so as to minimize immersion deposition, or to reduce it somewhat to avoid dissolution of photoresists. Similarly, quantities of the various components may be maximized to obtain higher overall conductivity for barrel applications or minimized to obtain greater fluidity for efficient pumping.
Deposits from neutral gold solutions are usually required to have excellent solderability and wirebonding capability, both of which are usually associated with softness and high purity. There are certain classes of brightening agents, referred to as grain refiners, that allow the maintenance of deposit hardnesses below 90 Knoop and purities above 99.9 percent while improving the overall reflectivity of the deposits and expanding the working range of current densities over which they may be obtained.
Thallium, lead and arsenic are examples of metallic grain refiners, often used in concentrations of about 1 to 3 ppm. Organic grain refiners include various polyamines and polyfunctional compounds. Thallium in solution concentrations greater than about 10 ppm has been implicated in embrittling wirebonds. Arsenic at concentrations above
30 ppm produces mirror-bright deposits with hardnesses above 140 Knoop from neutral solutions, but valence instability renders arsenic very difficult to control.
Acid Hard Golds
Acid hard-gold systems were the immediate beneficiaries of the realization that alkali -gold cyanides such as KAu(CN)2 were stable in solutions without free cyanide at pH values as low as 3.2. By operating in the pH range of 4 to 5, it is possible to incorporate transition metals such as cobalt, nickel and iron into alloyed gold deposits that are hard (130-200 Knoop), bright, ductile and, with suitable fluxing, solderable. Deposit compositions are 99.7 weight percent gold or higher, and both composition and physical properties can be maintained, even when plating in ranges of current density. The acid hard golds rapidly became the finish of choice for separable electrical connectors.
Acid hard-gold plating systems have been intensively developed for operation at very high current densities, also for uniform deposit distribution and for compatibility with various types of selective plating apparatus. Most current electrolytes are based on mixtures of weak organic acids and their salts. Depending on the choice of electrolyte, the metallic brighteners may be added in simple, complexed or chelated form, together with surfactants, range extenders and depolarizing agents, as needed.
Monovalent Gold Strikes
A strike solution is one in which the ratio of
crystallite nucleation to grain growth is enhanced by operating at higher-than-
normal current densities from a solution containing only a small amount of metal. The danger of dendritic growth, or burning, is minimized by keeping the current efficiency low
and the deposition time short. The result is a fine-grained, thin deposit of highly uniform distribution and excellent adhesion. Strike coatings are often used prior to heavy deposition from a conventional plating solution.
Because of stability considerations, monovalent gold-strike solutions are limited to a minimum pH of about 3.5, which is sufficient to obtain adhesion to nickel and alloys such as Kovar, but not to most stainless steels. The maximum pH is ordinarily about 4.5. As with the acid hard golds, electrolytes tend to be based on phosphates, weak organic acids, or mixtures thereof. Brightener systems are sometimes employed to allow heavier deposits, but this is unusual in strike solutions.
Trivalent Golds
The reaction of chloroauric acid, HAu(Cl)4, with an alkali cyanide yields the alkali gold (III) cyanide MAu(CN)4, where M is sodium or potassium. As mentioned previously, these species are stable at pH values down to almost 0. This allows the formulation of gold-strike solutions capable of activating and adhering to stainless steels. Somewhat similar solutions have been prepared using chloroauric acid itself. In practice, the cyanide-containing solution yields finer-grained deposits and is less subject to immersion deposition.
Trivalent gold-cyanide solutions also have been used in jewelry plating to deposit very bright, adherent layers up to about 5 micron thickness. Electrolytes for these processes operate typically over a pH range from about 2.5 to 3.0. Below pH 2.5, most organic acids are only weakly ionized, requiring additions of inorganic sulfates or chlorides to provide conductivity. Above pH 3.0, trivalent gold tends to revert to monovalent, particularly if organics are allowed to accumulate in the solution. This condition can be alleviated by destroying the organics with peroxide, but the peroxide has to be removed completely in order to restore current efficiency.
Noncyanide Gold Plating
Gold can be deposited from the chloroaurate. Various other forms, such as the iodide,
thiosulfate, thiocyanate and thiomalate also have been proposed, but have not been commercialized. The preparation of sodium gold sulfite, Na3Au(SO3)2, was reported in 1845, but commercialization of processes based on sulfite began only in the early 1960s. Since that time, sulfite-based processes have come into fairly wide use, particularly for semiconductor applications. More recently, there has been renewed interest in thiosulfate systems as well.
A sulfite gold-plating solution consists of the alkali gold sulfite, a conducting electrolyte and at least some excess of free alkali sulfite. The stability constants for sulfite complexes are lower than those for cyanides, and the excess is required to stabilize the gold complex. Sulfites are stable at alkaline pH. Addition of acid to sulfite ion releases hydrogen sulfite ion, and then sulfur dioxide. Since the anode reaction removes hydroxyl ions and thus tends to acidify the solution, it is common to operate sulfite golds at alkaline pH, usually 9.0 or above.
Brightening agents for sulfite gold systems have included arsenic, antimony and thallium. Recently a newer, stabilized series of sulfite golds have made it possible to operate at pH values below neutral. This has made it possible to employ cobalt, nickel, or organic polymers as brightening agents, or to operate without brighteners entirely.
Silver Plating
Whereas the complex cyanides of gold are stable at pH values low enough to allow the use of electrolytes without free cyanide, the complex cyanide of silver (only the monovalent species is formed) is unstable below neutrality, hydrolyzing to release insoluble AgCN. This imposes a requirement to maintain at least some free cyanide in the system, and sets a minimum operating pH for cyanide silver plating solutions of at least 8.0 to 8.5. Within the general class of cyanide silvers, there are numerous variations; optimized, variously, toward anode corrosion, deposit brightness, and plating speed.
Cyanide Silver Solutions
Typical general-purpose cyanide silver-plating solutions consist simply of about 90 to 120 g of free alkali cyanide per liter, together with about 25 to 40 g metallic silver per liter, added in the form of the corresponding alkali silver cyanide, or as AgCN.
At one time, the use of sodium cyanide was the norm, but this was largely superseded by potassium cyanide because of the higher solubility of most potassium salts. As a result of hydrolysis, carbonate gradually forms in alkaline cyanide systems, and at concentrations greater than about 90 g/L, sometimes less, impedes dissolution of the anode and causes deposit roughness. Excess carbonate can be “frozen out” from sodium cyanide-based solutions by chilling the solution to 3°C or so and filtering. This is not possible in potassium-based solutions, which require treatment with calcium cyanide or barium cyanide. Some brightener systems, particularly selenium or sulfur-based, are more effective in sodium-based solutions.
Free cyanide in a silver bath performs several functions. It solubilizes the silver, functions as the electrolyte and corrodes the anodes. As noted previously for gold, cyanide ion is highly surface-active and requires thorough rinsing after plating.
Carbonate is sometimes added at makeup to increase conductivity and to allow the free cyanide to be reduced somewhat; but since carbonate forms as the solution is worked, this is often dispensed with. Nitrate and alkali hydroxide have both been used to increase the corrosion rate of the anodes and, in the case of hydroxide, to retard decomposition of the cyanide as well. In any event, the anode efficiency of cyanide silver processes almost never actually achieves 100 percent, and it is periodically necessary to replenish a portion of the plated-out silver with alkali silver cyanide or with AgCN.
Brightening agents for silver include various compounds containing antimony, bismuth, selenium and sulfur. Most are proprietary. The sulfur-bearing materials in particular are often complex, being reaction products of various organic compounds with carbon disulfide, sodium thiosulfate or similar reagents. For this reason, they are often referred to as “organic” brightening agents, but in almost all cases, the active functionality derives from the sulfur.
Brightener systems for cyanide silver solutions almost always include both the primary brightener and a surface-active agent, the functionality of which is not completely understood. One possible mechanism would be for the surfactant to allow the primary brightener to adsorb in one particular orientation onto the crystallite surface.
Another would be to control the viscosity of the diffusion layer so as to regulate the access of the primary brightener to the surface. In any event, various surfactants appear to be optimal for specific primary brighteners, and in general, the effects of the added surfactants are most easily discernable at lower current densities.
Brightened silver deposits of all types are significantly harder in the as-plated condition than annealed, wrought silver. In most cases, the hardness of unannealed silver deposits decreases slowly over time after plating, presumably owing to recrystallization and grain growth. Antimony-brightened deposits, however, relax quite slowly over time, and are considered to be permanently hardened.
Electrolytes for high-speed plating of cyanide silver are of two, general types: 1) solutions containing appreciable amounts of free cyanide, in which replenishment of the silver is accomplished at least partially by anodic dissolution, and 2) solutions designed for use with insoluble anodes and containing only as much free cyanide as necessary to prevent precipitation of AgCN at the anodes.
Solutions of the first type are commonly used for plating wire and for overall or controlled-depth plating of electronic components. Solutions of the second type are used in applications requiring solution impingement or high solution velocity, as in spot or stripe plating.
Silver is electrochemically noble with respect to most other metals—even to gold in the cyanide system. Thus, it is highly prone to immersion deposit onto less noble surfaces, a condition which can be somewhat mitigated by maintaining a relatively high level of free cyanide.
Solutions of the high-velocity, low-free-cyanide type are very immersion-prone, particularly because they are commonly operated at high metal content and at elevated temperatures. It is common practice to employ a pretreatment to partially passivate the substrate surface prior to entering these solutions.
An overall silver strike, if permissible, is a useful alternative. Some solutions of the low-free-cyanide type also contain components designed to minimize immersion deposition. Cyanide silver strike solutions for general applications typically contain 2 to 2.5 g silver metal/L, together with about 90 to 105 g/L of free alkali cyanide. They are operated at ambient temperature and at relatively low tank voltage. For passive-prone metals, particularly for carbon steels, you may want to employ a mixed silver-copper strike solution followed by a pure silver strike prior to silver plating. A sulfamate nickel strike without chloride or bromide is an alternative to mixed silver-copper strike.
Noncyanide Silver Plating
Of the various soluble noncyanide forms of silver, only two have achieved commercial success in electroplating applications. These include organosilver complexes, of which the complex with succinimide is best known; and the alkaline thiosulfate, to which succinimide is added as an anode activator.
The design of electrolytes for noncyanide silver plating is constrained by the requirement to maintain the anodes in an active and film-free condition as a source of silver for replenishment. Nitrate, lactate and sulfamate are suitable for this purpose, and various combinations have been used. Also note that succinimide hydrolyzes slowly at the operating pH (generally from about 7.5-9) and has to be periodically replenished, regardless of whether or not the plating solution is actually being used. Since the hydrolysis process consumes hydroxyl ions, the solution pH falls slowly as well, and must be periodically adjusted.
However, deposits from the succinimide solutions are semibright, with good conductivity and above-average resistance to wear. In the as-plated condition, they are harder (120-130 Knoop) and more stressed than corresponding deposits from cyanide solution; but as with cyanide deposits, they show relaxation with time at ambient conditions, and can be annealed by post-baking. In the as-plated condition, they are more subject to tarnishing than deposits from cyanide, but this can be largely overcome by chemical treatment and stress relief, even by immersion in hot water.
More recently, a series of silver complexes with hydantoin and substituted hydantoins have been prepared and are coming into use. These promise improved pH stability and solution control, as well as higher brightness and greater resistance to tarnishing.
RELATED CONTENT
-
Nickel Electroplating
Applications, plating solutions, brighteners, good operating practices and troubleshooting.
-
Stripping of Plated Finishes
The processes, chemicals and equipment, plus control and troubleshooting.
-
Masking for Surface Finishing
Masking is employed in most any metal finishing operation where only a specifically defined area of the surface of a part must be exposed to a process. Conversely, masking may be employed on a surface where treatment is either not required or must be avoided. This article covers the many aspects of masking for metal finishing, including applications, methods and the various types of masking employed.