Non-Electrolytic Aluminum Oxide Conversion Coating
A non-electrolytic method can be used to produce a thin aluminum oxide coating of the same or better quality as an electrolytic conversion coating while using environmentally friendly and non-toxic materials...
The term "conversion coating," as used in the finishing industry, refers to the conversion of a metal's surface into a surface that will more easily accept applied coatings and/or provide for a more corrosion resistant surface. These coatings are rather thin (not more than 600 nm thick on aluminum), quickly and easily formed, easily scratched and, if used to enhance paint adhesion, coated shortly after application to prevent degradation of the conversion coating.
Conversion coatings for aluminum have been in use since the early 1920s, and there are a number of different products on the market. The four main types of conversion coatings are based upon: the production of a film of chromium hydroxides and/or oxides; the production of a film of precipitated heavy metal phosphates or oxides; the use of various synthetic polymers, with or without heavy metal phosphates or oxides; and the formation of a manganese oxide-aluminum oxide film by use of permanganates. Manganese oxide-aluminum oxide based conversion coatings are particularly useful since they have excellent barrier properties and greatly enhance the adhesion of applied coatings in general.
Electrolytically generated, or anodized aluminum, oxide surfaces have long been used to give the highest quality and range of aluminum oxide based conversion coatings. This quality comes at a cost in terms of time, money and effort. This article presents a less expensive non-electrolytic method to produce thin aluminum oxide coatings of the same or better quality using environmentally friendly and non-toxic materials.
Cleaning and Deoxidizing
In any metal processing procedure (painting, conversion coating, anodizing, etc.), the most important part of the processing is the proper cleaning of the metal surfaces prior to processing. Cleaning is the removal of surface oils and loose dirt. In general, alkaline cleaners do the best job. When used on aluminum they should be non-etching, since etching will leave difficult-to-remove alloyed elements, such as heavy metals or elemental silicon, and may pit the metal surface. To prevent excessive etching, silicates are usually added to alkaline cleaners. If added, they should be present in small amounts (generally less then 500 ppm), as excessive amounts will hinder cleaning and leave difficult-to-remove silicate deposits.1
Deoxidation is the removal of oxides and other inorganics that would otherwise interfere with further processing of the aluminum without significant attack upon the aluminum surface.2 To prevent excessive attack, deoxidizers generally contain an oxidizing agent designed to maintain a thin film of oxide on the metal's surface. This allows for the oxide to be removed rather than having a direct attack on the metal by the deoxidizer.
Featured Content
Many of the deoxidizers now in use will use an iron (III) salt, such as ferric sulfate coupled with hydrogen peroxide,3 or any one of a number of different oxidizers (chlorates, nitrates, persulfates, etc.). Iron based deoxidizers leave deposits of iron on the surface of the aluminum that encourage galvanic corrosion, since you have two dissimilar metals in direct contact with each other. The other oxidizers mentioned are, in general, not strong enough oxidizing agents to maintain a good oxide film on the metal or have toxicity problems associated with them. The best deoxidizers for aluminum are those based on nitric acid coupled with another oxidizer, such as hydrogen peroxide or sodium bromate.4 Unlike other acids, nitric acid will dissolve aluminum oxide but has very little effect upon aluminum itself.
With high silicon content alloys, it is difficult to avoid the use of fluorides in spite of their toxicity. If you must use fluorides, keep the fluoride level low (generally not more than 200 ppm) as excessive amounts of fluorides will leave a white deposit of insoluble aluminum fluoride on the metal's surface and will pit the metal. The use of acids other than nitric and the use of heavy metals, such as iron, will have the same effect.
For years chromic acid and/or chromates were used in deoxidizers in conjunction with nitric acid and were generally considered the best deoxidizers on the market. In addition to the toxicity issue associated with the use of hexavalent chromium, these deoxidizers always leave a thin deposit of chromium oxides on the metal's surface that will not allow for the subsequent use of a non-hexavalent chromium conversion coating system.
Non-Electrolytic Conversion Coating Process
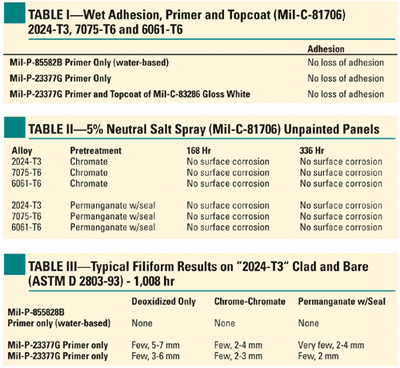
The first stage of the process is the formation of a hydrated aluminum oxide film by the use of boiling deionized water. The aluminum begins to react within 10-15 sec and completes the formation of a 30-40 nm thick, soft, blue-gray coating in about 5 min (see Figure 1).
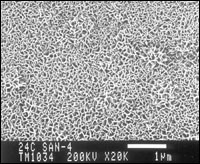
The second stage of the process involves treatment of the aluminum in a proprietary aluminum salt solution at about 200F for at least 1 min to decrease the hydration of the aluminum oxide film and remove unwanted inorganics (smut). At this point, the aluminum is metallic in color and has a well-defined structure (see Figure 2).
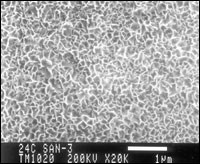
The third step involves treatment in a proprietary potassium permanganate solution at 130-140F for at least 3 min to create a manganese oxide-aluminum oxide coating of about 30-40 nm thick with a very well defined structure (see Figure 3). The metal itself will have a clear to light gold, iridescent finish. The coating is quite hard and scratch resistant, will withstand temperatures up to the melting point of the aluminum and will not degrade over time.
The already excellent corrosion resistance and paint adhesion may be still improved further by use of one of several secondary seals available for that purpose. The secondary seals are non-toxic, environmentally friendly and water-based products applied at ambient temperature and allowed to air dry. Potassium permanganate is on the list of materials allowed in potable drinking water5 and has been used for more than 80 years in the treatment of potable drinking water systems. Potassium permanganate will leave no heavy metal residues in your rinse water.
Paint Adhesion and Corrosion Resistance
Numerous paint adhesion and corrosion studies have been performed on bare and painted aluminum alloys conversion coated by this process.6,7 Bare salt spray corrosion resistance according to ASTM B-117, filiform corrosion resistance studies and paint adhesion studies are presented in Tables I, II and III. In all cases the coating is shown to meet or exceed the required standards of the tests. Independent tests on high-solids, heat-resistant paints have shown performance values that exceed those of chromic acid anodizing in paint adhesion and corrosion resistance characteristics.
Hexavalent chromium based conversion coating systems have been used for more than 60 years because they have provided such excellent corrosion resistance and paint adhesion characteristics when used with aluminum and its alloys. Any replacement must be designed to duplicate its characteristics as closely as possible. The manganese oxides produced by the heptavalent manganese based system are by far the most closely related to the chromium oxides and hydroxides in terms of their respective chemistries. Thus, the manganese oxide-aluminum oxide film, as produced by the heptavalent manganese conversion coating system, is the most closely matched in terms of performance and actual chemistry.
To learn more visit Sanchem, Inc..