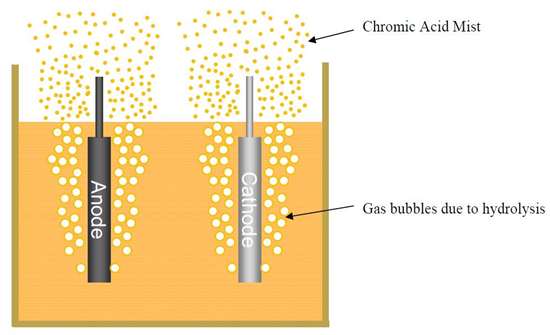
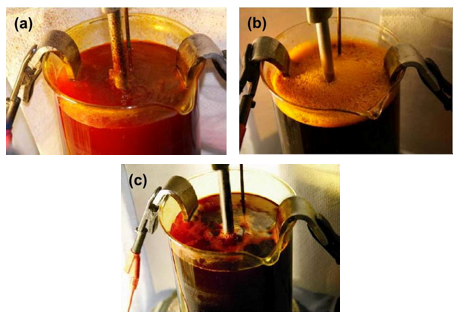
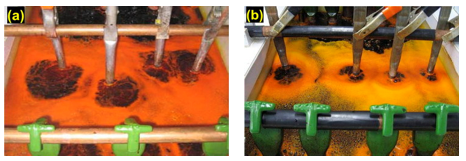
The test was performed in a fume hood and the surroundings covered in paper to prevent contamination. It is easy to see that the beaker, cathode rod and protective paper are heavily contaminated with chromic acid due to the mist that has been generated (also, Fig. 4(a)). This is after just five minutes of plating. Reduction of the mist can be made in several ways, but a popular method is to employ a mist suppressant.
When either a foaming (Fig. 4(b)) or a low-foaming (Fig. 4(c)) mist suppressant is used, then contamination to the surroundings is significantly reduced. The beakers and surroundings tested with each version of mist suppressant showed no evidence of contamination even after several hours of plating.
Most common mist suppressants are surfactant-based and work by reducing the surface tension of the solution. This has a two-fold effect on the generation of mist. First, a reduced surface tension reduces the size of the gas bubbles generated during electrolysis. These smaller bubbles travel slower through the solution and have less energy when they arrive at the solution’s surface. Second, at the same time, the lower surface tension reduces the energy with which the resulting droplets are ejected into the air. Together, both of these effects can reduce the emission of droplets, and therefore mist generation, by over 98%. When a mist suppressant is properly applied, this has been found to be sufficient to reduce exposure to employees around plating installations.
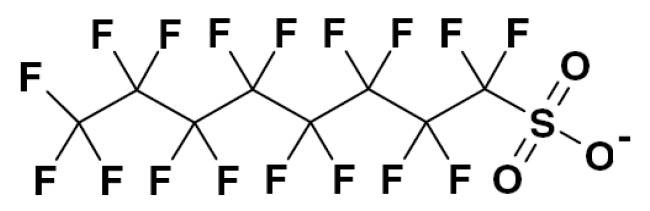
Due to the aggressive chemical and electrochemical environment of chromium plating solutions, most mist suppressants are made from highly stable surfactants. Perfluorooctanesulphonate (PFOS) (Fig. 5) was, and still is, commonly used as the basic surfactant in popular mist suppressant products.
PFOS is highly resistant to chemical attack and so is ideal for use in harsh environments like hot chromic acid plating baths. Unfortunately the extremely robust nature of PFOS means that it is not easily biodegraded or waste-treated and so is released into the environment where it builds up. PFOS has been classified as a persistent, bioaccumulative and toxic (PBT) for some years now.
The negative health effects of PFOS on animals and humans have been studied and were found to be unacceptable.
1,2 As a result, there are several legislations worldwide restricting the marketing, production and sales of substances, including PFOS. In the U.S., the EPA
3 included PFOS in a new table of substances now subject to Significant New Use Rules (40 CFR 721.9852). This rule became effective on November 11, 2007. There is an exemption for one form of PFOS, with CAS number 56 773-42-3, when it is used for metal finishing and electroplating applications, including hard and decorative chromium plating, chromic acid anodizing or reverse etching, etching plastics prior to metallization and nickel plating. There is a more restrictive PFOS directive from the EU
4 that came into effect on June 27, 2008. Further, there is a worldwide directive from the Stockholm Convention of Persistent Organic Pollutants, effective August 26, 2010, which affects over 150 countries including Japan, implementing an effective ban of sales and production of all PFOS-containing products starting April 1, 2010.
One of the main aims of these directives is ultimately to ensure the phase-out of the utilization of PFOS. There are currently exemptions in most of these directives for hard chromium plating.
5 However, there are several communities, organizations and authorities that are campaigning for a complete ban on PFOS use.
Due to the increased awareness of the HES risks, the use of PFOS has declined by over 95% in the last decade, leaving only a few specialized applications. As the health and safety implications of the use of fume suppressants for Cr(VI) applications are serious, a suitable replacement for PFOS must be rigorously tested to ensure its suitability. Investigations into a large variety of alternative, non-PFOS candidate compounds have been going on for several years.
Typical criteria for a good mist suppressant are:
· Reduce surface tension to ≤ 30 dynes/cm
· At 30 dynes/cm, have no discernible mist
· No effect on plating quality or efficiency
· No effect on bath stability
· Be relatively stable - minimal dosing
· Any breakdown products should not influence the bath quality or functionality
· No PFOS should be generated or created
Not all candidates are successful even if they seem initially to meet most of the criteria listed above. The unsuccessful candidate shown in Fig. 6 gave insufficient mist suppression, despite having a supposedly good surface tension of 29 dynes/cm.
Permanent and non-permanent mist suppressant candidate compounds were tested and it was found that the non-permanent mist suppressant compounds were basically unsuitable for use in the field. Most non-permanent, non-PFOS mist suppressant candidates that were tested failed the functional test as per the criteria noted above, but even those that passed most of the criteria, failed due to bath contamination.
Typically, the non-permanent, non-PFOS compounds were inherently instable in standard hard chromium plating solutions and thus required constant dosing, even during idle times. When the bath was under plating conditions, even more dosing of the suppressant chemistry was generally needed. This can cause control issues and needs constant monitoring of the dosing equipment to ensure that the maximum surface tension limit is never exceeded.
Apart from this, the biggest issue seen with this type of suppressant chemistry was the tendency for the excessive breakdown of the chemistry to form an “oil” or “scum” on the plating bath surface that eventually interferes with the plating quality.
The candidate tested as shown in Fig. 7 shows a more extreme example of this “oil” or “scum” formation. It is easy to imagine that parts plated in a solution containing this compound, with its surface “scum” layer, would cause plating quality issues.
After extensive testing of suitable permanent, non-PFOS fume suppressant candidates, a family of products was finally developed, thoroughly field tested and brought to the market. There are two versions of permanent, non-PFOS mist suppressants that have been developed and are available on the market today. One is the foaming type (Fig. 8) and the other, a low-foaming type (Fig. 9). Both are based on similar non-PFOS technology and have years of proven usage in the industry. These products can fully comply with PFOS legislation.
The low-foaming version of the fume suppressant is, in general, more popular due to the lack of a thick foam blanket covering the entire surface of the plating solution. This helps to minimize the issue of hydrogen and oxygen gas getting trapped in the foam blanket, which can lead to hydrogen explosions. It also means there should be less chance of foam being “sucked” into the exhaust system on tanks with minimal free-board.
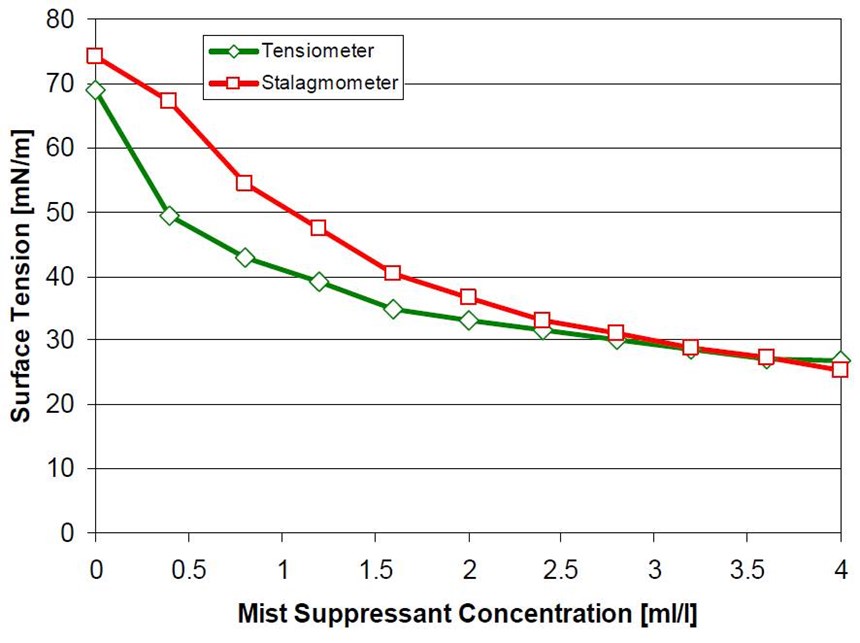
Comparison tests between current, leading PFOS products have been made, and the newly developed non-PFOS products performed just as well (Fig. 10).
The preferred method for ensuring the best operation of a fume suppressant in a plating bath is to monitor and control the surface tension. The permanent, non-PFOS mist suppressants can easily be monitored and controlled by either stalagmometer or tensiometer surface tension measurement (Fig. 11).
The surface tension of a chromium plating bath can be reduced to 20 dynes/cm using the non-PFOS products, but is commonly kept at an average of 30 dynes/cm. At this level, consumption of the mist suppressant is kept to a minimum and the Cr(VI) emissions are kept under control.
When changing from a PFOS-based product to a non-PFOS product, there are two options: a completely new make-up or a slide conversion. Both methods have been employed and studied. The most common method is to perform a slide conversion of an existing solution. This is generally an easy operation. The surface tension of the bath is analyzed and the required amount of non-PFOS product is then added to attain the desired surface tension. The non-PFOS product is subsequently dosed to maintain the surface tension within the desired range.
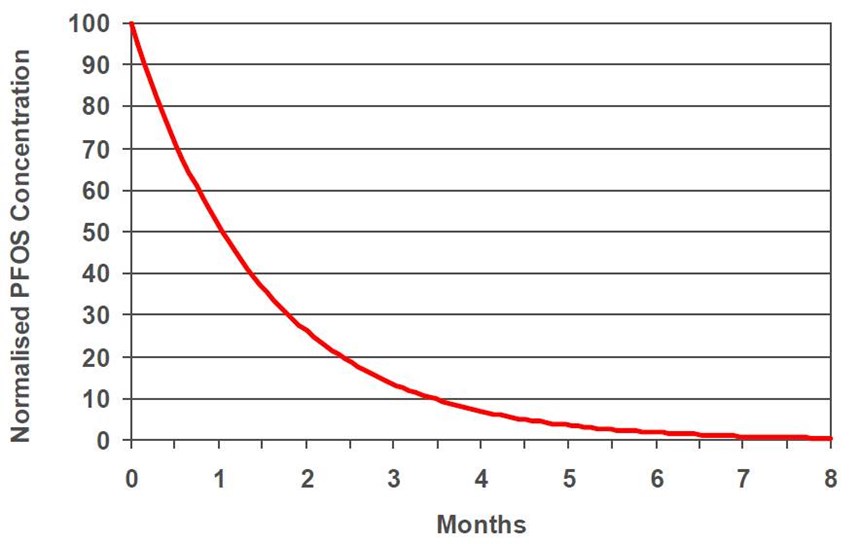
When a slide conversion is made, under normal circumstances, the PFOS concentration in the solution should reduce over time. One example of a slide conversion (Fig. 12) shows the potential decline in PFOS over time. This was a decorative chromium bath that was converted to a foaming type non-PFOS mist suppressant.
It took seven months to reduce the PFOS content to only 1% of the original level at this particular commercial plater. Different installations may yield different results due a number of factors.
In conclusion, there are now suitable, successful and well proven permanent non-PFOS mist suppressants available in the market, both foaming and low-foaming, for hard chromium and decorative chromium plating as well as chromic acid etching.
References
4. EU directive 2006/122/EC amending for the 30th time the council directive 76/769/EEC, 2006; http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:372:0032:0034:en:PDF.
5. Annex 3(c) of EU directive 2006/122/EC, 2006.
* Corresponding author:
Gene Barlowe
Atotech USA Inc.
1750 Overview Drive
Rock Hill, South Carolina, 29730-2000
Phone:
(803) 817-3500