The Challenge Before Us
This paper is a re-publication of the 3rd William Blum Lecture, presented at the 48th AES Annual Convention in Los Angeles, California, on June 19, 1961. Dr. Charles Faust discussed the challenges to the surface finishing industry in the wake of the massive growth in technology in the mid-20th century.
by
Dr. Charles L. Faust
Featured Content
Recipient of the 1960 William Blum AES Scientific Achievement Award
Originally published as
Annual Technical Proceedings of the American Electroplater’s Society, 48, 17-23 (1961).
Editor’s Note: This paper is a re-publication of the 3rd William Blum Lecture, presented at the 48th AES Annual Convention in Boston, Massachusetts, on June 19, 1961. A printable PDF version is available by clicking HERE.
To be chosen for an award by one's associates who have similar interests is an especially moving honor. I feel that a person standing in this spot as I am today has been given a "poetic license." To the poet, this means to coin new words to express his thought regardless of the pedantics of language. To me, this means to express a challenge regardless of habitual thinking and established practice, with the sole hope of being constructive.
Thoughts of subjects on which I might talk reminded me that my activities always have been connected with searching for new processes and products; and with encouraging industry to use new techniques and new concepts.
You, the members of the AES, have been most helpful in providing encouragement and follow up. I am most grateful for that. Throughout the half-century history of the AES, you have increased the knowledge of how to electroplate for exceptionally good protection and decorative beauty. And, you know ways to do this with good reproducibility in large and small lots of parts in daily production.
Research has given us a clearer understanding of which are the important details of electroplating processes as to influence on properties and performance of plates; has shown that surface cleanliness and preparation of the basis metal influence properties of the plate; and has improved the durability of electroplated articles because of better understanding of how electroplates protect.
Therefore, you may well ask, "What is the challenge before us?" On the one hand, it is to provide the means for fullest use of the knowledge now available to us for applying high quality electroplates. On the other hand, more meaningful ways are needed for specifying quality. To do this we can start with what we have now.
I had planned to show changes in finishing and plating specifications, as they have been evolved through research. I soon discovered that to do so would put me in the position of criticizing, which I have no intention to do. Nevertheless, our problem is revealed by reviewing specifications. Plating of zinc die castings will serve for illustration.
The most recent specification (1958) for copper, nickel and chromium plating points out, "The conditions of exposure and use of plated zinc are so varied that it is not possible to predict the average life of articles plated in accordance with Type FZ, Type KZ or Type QZ, or to predetermine which type of plating should be specified for a given article. Such a selection must be based on the experience of the manufacturers and users."
Thicknesses are given for the three types of plates, as based on tests and experience with buffed Watts nickel, which is seldom used for decorative-protective finishing now. We are informed that, "If the conditions are altered, different thicknesses may be required to give equivalent performance." Natural questions arise, "What specific guidance is disclosed?" and "What performance is expected?"
The specification states that average life cannot be predicted, and points out that continuity of coatings is an important characteristic. The manufacturer and customer shall agree on a period in salt spray without showing corrosion on significant surfaces. This is the implied measure of continuity.
Then a footnote adds that different salt spray cabinets operated in accordance with specifications number (which is given) failed to give reproducible results on replicate panels. Even so, there are instructions for preparing samples for this noninformative salt spray. So, what real, clear-cut guidance is given?
I am mindful of the great effort in time donated by industry representatives to prepare the recommendations and specifications. They have labored long and well. And, their efforts have done much to lead from chaos to order.
This order has provided for quality improvement as shown by recent publications. And, electroplates can be applied to give the outstanding protection demanded by industry.
This is illustrated by data taken from the AES Proceedings and Plating magazine issues of the past two years, as charted in Figs. 1 to 4. Figure 1 shows the extent of protection to be expected if any of three plating schemes would be used on zinc die castings. Clearly, the properties of the nickel plate and the preparation of the zinc die castings are critical factors.
If a rating of 9 is the cutoff point for acceptability, only one of the products will be acceptable and it will be in only the mildest of the three exposure sites. Further reading and/or study is needed if different performance is required. This will show that the character of the nickel and of the chromium plates is very important in setting protective quality.
From published information, Figs. 2 and 3 can be plotted to show the benefit from providing certain properties in plates as well as from having adequate thickness.
According to Fig. 2, a duplex nickel would be selected as the best of the three types of nickel plate. According to Fig. 3, a decision can be made as to thickness of the duplex nickel. Other data can be taken from the literature to show, as in Fig. 4, that a choice exists as to which chromium plate should be used over the selected duplex nickel.
Information can be dug out of the literature for similar charts to assist in deciding on which plating systems to choose for a given need.
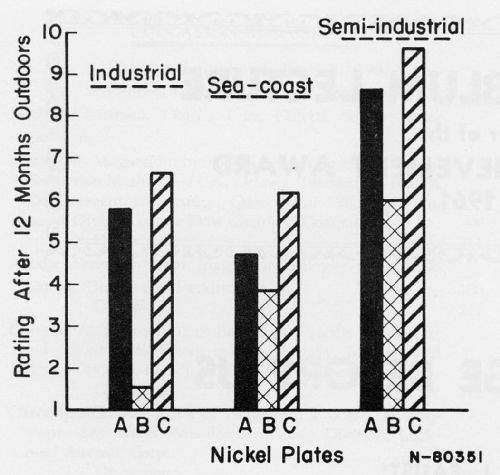
Figure 1 - 0.4 mil Cu + 0.8 mil Ni + 0.01 mil Cr on zinc die-castings: A - a bright nickel; B - buffed Watts; C - same plate as A with different basis preparation. From: W.H. Safranek, H.R. Miller and C.L. Faust, AES Technical Proceedings, 46, 133-140 (1959).
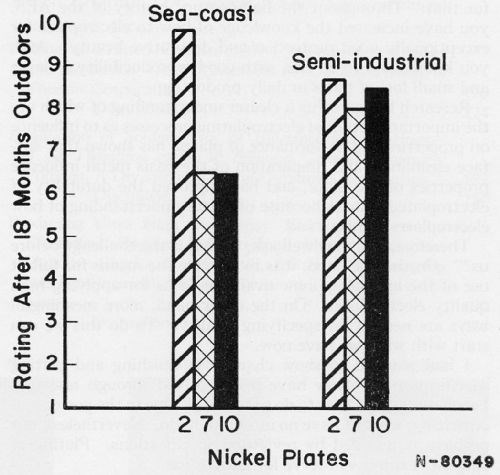
Figure 2 - 1.2 mil Ni + 0.015 Cr on Steel: 2 - a duplex nickel; 7 - a semibright nickel; 10 - a bright nickel. From: A. DuRose, AES Technical Proceedings, 47, 83-89 (1960).
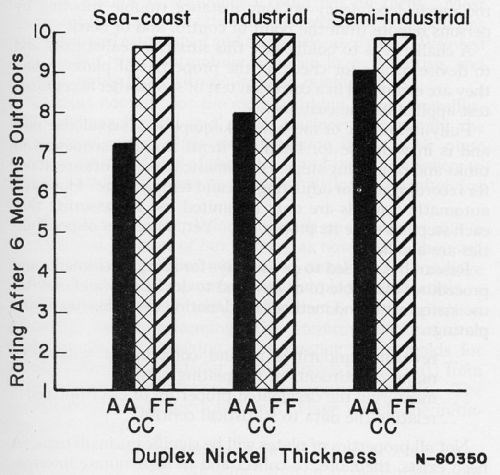
Figure 3 - 0.4 mil Cu + duplex Ni + 0.01 Cr on zinc die castings: A - 0.4 mil Ni; CC - 0.8 mil Ni; FF - 1.2 mil Ni. From: W.H. Safranek, H.R. Miller, R.W. Hardy and C.L. Faust, AES Technical Proceedings, 47, 96-104 (1960).
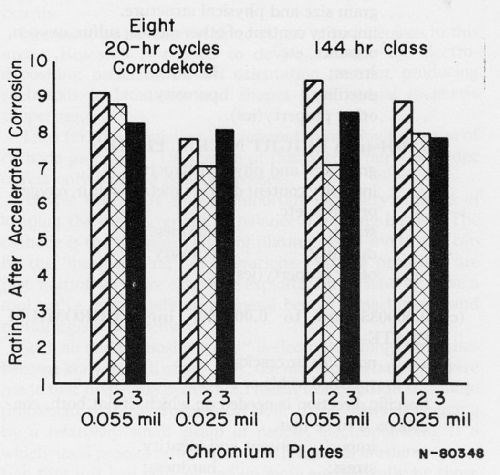
Figure 4 - 0.4 mil Cu + 0.8 mil duplex Ni + Cr on zinc die castings: 1, 2 and 3 are different Cr plates. From: C.L. Faust, G.R. Schaer and D.E. Semones, Plating, 48 (6), 605-612 (1961).
Therefore, because of research, appearance and durability of plated die castings are better than ever before. Yet, further improvement is necessary. Note from the four preceding figures, that the kind of electroplate is equally or more important than the thickness of electroplate. Inherent properties as well as external forces influence corrosion behavior of electroplates.
Operating under a policy that emphasizes only thickness and corrosion inspection, keeps electroplating and metal finishing in the category of being process oriented. We are at the threshold of a significant change.
Our challenge is to electrodeposit quality into electroplates. For fullest return on skills available to us, we must become more strongly product oriented. This may be accomplished by regarding and by using electroplates as materials for industry.
Publications by the AES and its members show much information on the following aspects for most of the electrodeposited metals we use now.
1. Influence of bath type, composition, and operating conditions on the properties of electroplates. The properties reported are:
- Crystal structure and size
- Porosity
- Hardness
- Ductility
- Stress
- Tensile strength
- Yield strength
- Fatigue strength
- Youngs modulus
2. Influence of impurities in plating baths and in electroplates on the above-mentioned properties. Data in this area need to be greatly expanded.
3. Influence of porosity on protective merit in corrosive media.
4. Types of plating bath to give the best covering power; and throwing power in micro and macro scale.
5. Ways to examine electroplates for:
- Porosity by electron microscope, electrographic printing, electrode potential measurement, etc.
- Physical properties by established practices of metallographic examination and mechanical testing.
- Chemical stability by exposure tests and by electrode potential measurements.
6. Thickness needed to overcome gross porosity as shown by accelerated and atmospheric exposure tests.
- However, the information is usually not correlated with specific properties or with precise details of electrodeposition.
This truly fine supply of information is available "cafeteria style." It is there for those who can and will help themselves. Procedures and results are described. However, the user has to recognize his own needs; often from conflicting reports and without complete guidance on what to select. Or, too much information is crowded into noncritical texts.
In all this information, there is very little correlation between properties of electroplates and predictable performance in particular uses.
The four figures referred to in the preceding discussion show what can be done when electroplates have the right properties. The data do not show what those properties should be, even though much research has been done on properties of electroplates.
Consider nickel plate, for an example, and for which Table 1 shows the ranges of properties attainable. As we know, other properties are critically important, such as: impurity content, porosity, grain size and structure, etc.
Table 1 - Properties of nickel plate according to plating bath type.1-5
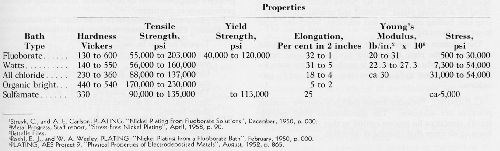
What particular properties should the bright nickel and the semibright nickel have in the very promising duplex nickel plate for good protection and appearance after 1 year, or 2 years, or 3 years, or longer outdoors? Research should pin down information for answering this question. And, do the same for copper, chromium, and other plates.
We must assemble metal finishing and electroplating data we now have for interpretation to direct instructions on how to electrodeposit predicted properties into electroplates. Research will be needed to fill in important gaps.
Also, research is needed for devising ways to determine that the plate is maintaining properties level while loads are being plated, and immediately thereafter. Then the plater can know that he has met the quality requirements for predictable performance.
New specifications should be prepared with direct guidance to quality. They will name properties electroplates must have. Imagine such specifications for finishing zinc die castings.
Acceptability for plating
A specification is needed for defining the surface condition the plater will accept, in terms of:
- Thickness and structure of dense skin
- Amount and kind of porosity, if any, that will be tolerated.
- Amount of cold shut, if any, that will be tolerated
- Condition of parting-line trim and finish there
- Other factors that will influence quality of electroplate.
Procedures for checking acceptability of die casting for electroplating.
- Need studies to define methods for porosity and cold shut inspection; may be electropolishing or chemical milling.
- Need studies to define means for showing 'width/depth' ratio of pores and holes that will be accepted
- Other needs will become apparent.
Preparation for plating
Polishing
Include or limit to specification of non-mechanical finishing method. This avoids smearing over pores and holes or cold shut areas to cover but not seal them shut. A double advantage is gained: (1) inspection and (2) opening of holes and pores so the “width/depth” ratio allows filling by a leveling plate. Covered and unsealed pores, etc., trap oils, buffing compound and solutions, which can cause in-service blistering.
The influence of a non-mechanically finished surface on the structure of the plate is generally favorable toward better quality electroplates. Research is needed to define ways for determining sound surface preparation and to certify procedures.
Cleaning
Presently known mild, but effective cleaning practices are adequate. The specifications must provide specific directions for process control so that the operator can know at all times that cleaning is optimum.
Specification for plates
This is an area in which the greatest amount of effort is needed. Some of the needs can be answered by data in the literature. The rest must be provided by experimental programs to fill the blank spaces in a specification that may be like the following for bright protective-decorative plating:
(a) 0.0004-inch copper plate:
- Grain size and physical structure.
- Impurity content of other metals, sulfur, oxygen, organics, etc.
- Leveling action expressed as a measurable quantity.
- Stress
- Hardness
- Ductility
- Porosity
- Other property (ies).
(b) 0.0012-inch duplex nickel plate:
0.0008-inch semibright leveling
- Grain size and physical structure
- Impurity content of other metals, sulfur, oxygen, organics, etc.
- Stress
- Hardness
- Ductility
- Porosity;
- Other property (ies).
0.0004-inch bright nickel plate
- Grain size and physical structure.
- Impurity content of other metals, sulfur, oxygen, organics, etc.
- Stress
- Hardness
- Ductility
- Porosity
- Other property (ies).
(c) 0.000035 [up to 0.000050] inch chromium plate
- No voids or cracks.
- Specified voids or cracks.
Specific direction is needed on which, if not both, condition is to be met.
- Impurity content and identity.
- Stress
- Hardness
- Other property (ies).
The above is merely an example. Thicknesses are shown for illustration only, but are in accord with the good results of Figs. 1-4.
Electrodeposition methods
This may be the toughest of all to declare. Habit, prejudice, and proprietary interests are involved. However, any electrodeposition procedure that gives electroplate with the specified properties obviously can be adopted. This is why property designation can be so valuable in attaining superior performance in the future.
For plates with properties to be cited under "Specifications for Plates", electrodeposition methods must stipulate:
- Type of bath to be used.
- Concentration ranges and ratios for principal constituents.
- Identification of and defined concentration limits for impurities.
- Throwing power and covering power as statement of depth and area of recesses relative to total area and any shape factor.
- Temperature, acidity (or alkalinity); surface tension. . . . Suspended matter control.
- Type and control of anodes, as by bagging or using diaphragms
- Other factors as may require designation.
Although I have used zinc die castings as an example, the challenge applies to all basis metals and electroplates. Even without property specification, we can see the intrinsic values of electroplates. Three to five years and longer without deterioration is attainable by techniques now in use. For assurance of always getting the best results, we need only to identify these best plate properties and plating techniques, and specify clearly how to keep them "On the beam."
Perhaps my stand is toward professionalism. And, why so? We are on the border now. Let us face some obvious facts. Metallurgists, chemists, engineers, with whom we associate in our work, are looked on as professional men. They are product and materials oriented. This is a near goal for metal finishers and electroplaters.
Electroplating and electrodeposition have passed the black art stage, long ago. However, control of processes and choice of processes for specific results as a material are from too great a distance. Still too much rule of thumb; too much scrambling to correct trouble after it has happened or because of rejects; too much dependency on long distance trouble shooting by persons remote from the point of control and of need.
A challenge is to boldly face this situation realistically and to devise means for checking the properties of plates before they are evaluated in a corrosion test or some other acceptance test applied by the customer.
Fully automatic or mechanized equipment is available now and is in wide use for handling items through sequences of tanks and processing steps. Automatic instruments are in use for recording and /or adjusting pH and temperature. However, automatic controls are only in limited use for assuring that each step has done its proper job. Yet, a number of possibilities are available.
Research is needed to devise ways for using instruments and procedures available to us now and to devise new and easy-to-use instruments and methods for denoting the following during plating:
- Revealing impurities in and composition balance of major constituents of the plating baths
- Measuring the designated properties of electroplates
- Relating the data to statistical control.
Not all properties of plates will be significant in all uses. A need exists, therefore, to collect and to disseminate information on which properties are pertinent to particular applications. Filling these needs would be in preparation for larger fulfillment of using our skills. The AES is the logical organization to undertake the task.
Perhaps I am walking where angels fear to tread. However, I feel so strongly that electrodeposition has so many more values than we're giving and receiving in the way of benefits and utilization, that I must go on.
Any action such as I propose will be met with opposition, which feels there are good reasons for doing nothing. Yet, we have to discard some of our comfortable routines and replace them with new ones. This is an overburdening challenge for those who have psychological barricades.
One such barricade we hear frequently is, "We have not yet proved conclusively that the methods we're now using can't do the job or that the proposed new methods can." Another we hear is, "The time is not ripe."
Such challenges as put forth herein undoubtedly will spark controversy. It is easy to give good reasons for ducking the issues. The reasons sound so convincing.
By increasing the tempo of thinking of electroplates as materials for industry, and providing definitive specifications for electrodeposition as a way to produce materials or to effect new accomplishments, we shall expand the horizon for new products and new business.
You might ask, "Are materials within our scope?" I believe the answer is yes. To take up this challenge may require enlarging the fields of interest of the AES. If we, the members of the AES, do nothing toward that accomplishment, another society will, or a new society will form and take the initiative we can take now.
In the foreword of "The Challenge of the Materials Age" (Materials in Design Engineering, Special Report No. 175, September, 1960), we are advised to reappraise our way of doing things. "Our way" refers to industry at large. But, it also applies to that segment represented by the AES. What is the impact of this thought on the AES? The opportunity is here for initiative and expansion.
In electrodeposition, we have the process for microscopic genesis of materials. Metals are produced through building atom-by-atom, starting from nothing and producing a plate on a substrate, a sheet, a strip or a product shape (an electroform). This we know and practice now. However, we have only a foot in the door of what can be accomplished.
Table 2 reminds us of some of the properties of electrolytic metals in comparison with metals produced by classical thermal processes. On a property basis, the electrolytic metals can compete in many uses. The blank spaces are there because in 4 hours of searching I could not fill them. Most significant to us is the fact that the electrodeposition conditions have been well established and are widely used now. We need only to put the information together in a definitive and directly usable form with no hedging qualifications.
We must not wait for the metal working people, metallurgists, and mechanical and design engineers, to supply the imagination out of their own desperate needs. We must increase our knowledge of their needs and take the answers to them. This is being done now; but too little and too often too late.
Materials have never before had such an important role in the technical progress of our country as now and as they will have in the future. Electrodeposited metals and alloys have just barely started to assume a rightful place as materials.
We are all familiar with utilization of electrodeposition knowledge for electroforming. Our literature discloses some truly fine work in making items ranging from: molds for plastic trinkets to molds for large radomes for aircraft; from bases for dental restorations to wind tunnel Venturis. The aerospace age needs electroforming. Have we left the initiative up to others?
Table 2 - Properties of electrolytic metals in comparison with other materials.
Have there been too many instances of nonsuccess because of a "quick look" or a "one-time" trial on an inadequate foundation of knowledge and know-how? Quite possibly. Here is a field still untapped for electrodepositors with know-how, skill and imagination. Members of the AES have opened the door, but only a crack.
Our challenge is to know when electroforming should be first choice and to discover the way to exert influence at the design and production planning stages. This will come easily just as soon as electrodeposits are widely looked on as materials produced to property specifications.
Many metals-joining jobs are very difficult and expensive to do. This is because heat and/or pressure cannot be used; for any one of several reasons:
- The metal properties are sensitive to heat.
- The metals oxidized too rapidly.
- The sections are too thin.
- One or both metals distort when heated.
- No suitable flux is available.
- Low melting alloys form to cause weak joints.
Quoting [again] from “The Challenge of the Materials Age” (loc. cit., page 149), "Our weakest link is how materials can be worked, formed, and joined - particularly the high-temperature metals, ceramics, and cermets."
Electroforming and joining or welding by electrodeposition can be ideal answers to one of these weakest links. No heating, no distortion, no pressure is involved in the method of joining by electrodeposition welding. Another challenge is to provide the techniques to our members for their business good and for the good of space materials needs.
Because of good engineering properties, electrometals have an important role in cladding. With electroclad metals, the materials engineer can use the best properties of two or more metals. My first industrial research task over 25 years ago related to producing a material by electrodeposition - a bearing alloy liner of cadmium-silver. The alloy, up to 0.06 inch thick on flat steel back provided a clad material that could be formed to half-shell shape.
Electrocladding is another technique we should be exploiting more extensively, as another practical and important use for our skills. Important accomplishments are indicated by papers on electroplating on unusual metals or electrocladding structural materials.
Magnetic materials are interesting prospects by electrodeposition and are being produced. Thin strip is easier to electroform than to produce by rolling. This we also know because we have made electrolytic foils for years. Now electro-copper foil is the best material for making printed circuit boards.
However, we have left it up to others to be ingenious in this area. Research is needed to devise methods for electrodepositing preferred crystal orientation and for producing such foils in laminates and shapes with special magnetic properties.
Thus far, the discussion has referred to making fuller use of cathode processes. Utilization of this part of our knowledge covers only half the skills we possess.
Anodes take part in the important auxiliary process of keeping the metal content in balance in plating baths. The cathode is the "business end" of plating. The anode also can be the "business end" of operations. Anode processes are just waiting for more extensive exploitation. Here, we have a tool we've largely left up to metal benders, machinists and metallurgists to discover.
Such an anode "business end" is electropolishing, which has become known to all of us. Yet, the most important uses were made first by people with whom metal working skill was more prominent than electrochemical skill. After years of plugging by a relatively small group of people, electropolishing is a widely used process now. Yet, here is a metal finishing operation that if it had been picked up more energetically by those experienced in electrolytic procedures would be much more useful than it is.
We have come a long way from finishing armor in an ale keg with court-yard stones and a serf rolling the keg around the countryside, uphill and down to get more action.
The din must have been terrific. Probably the operation is the forerunner of the term "Rattling" in reference to modern barrel finishing, or tumble finishing, and is the dark-age ancestor of flat polishing, automatic machine polishing and buffing, and bright rolling.
All have one aspect in common: They tamper with and alter the surface. The surface only is involved. The inside or bulk of the metal (or material) is undisturbed.
Witness the account of barrel finishing eggs (W. Biebel, Plating, 45 (1), 31 (1958)). This one intrigued me. Facetiously, I wondered if a barrel finished egg would be better fried, poached, scrambled or hardboiled. Seriously, the discussion showed how gentle finishing can be to delicate items.
Nevertheless, some work is done on the surface. We in the metal finishing industry must always recognize this situation and consider what is the effect property-wise on performance of a product in its intended service, with or without an electroplate. Also, what effect if any does the surface state of a metal acquired during preparation have during a plating operation on the properties and performance of the final product.
To move forward according to proposals made herein requires instilling metallurgy as well as chemistry into electrochemistry. Some aspects of this have been touched on in discussions I have called “surface metallurgy” to which I refer you for further thinking.
This is an important factor in these days of ultra-high strength steels, special high-temperature alloys for nuclear and aerospace and even for more customary uses of decorative-protective finishing.
Nonmechanical methods have an important role in metal finishing after some operation has plowed, pushed around, pounded, or cut the surface, i.e., has imposed a handicap on use or subsequent processing. Finishing methods have risen to the occasion. In spite of the tampering done with the surface in machine shops, press rooms, foundrys, etc., the finishers have ways available to provide the improved properties demanded at a cost that can be accepted.
Now with the technology available to electrodepositors, or more appropriately electrodissolvers, we can take a large step ahead and provide another service to industry. I refer to electrolytic machining, shaping, grinding, or by whatever name it will become known. To too great an extent we have left the field up to nonelectrochemical interests - machine tool builders, machinists and mechanical engineers. Much of the work has the aura of "fitting the foot to the shoe"; attempting to install electrochemical operations on machines designed and intended to do mechanical jobs.
The opposite approach is needed. Apply the electrochemical principles we have on metal treatment first and design the machinery second. This has been done. Recent work on this subject is the outgrowth of attempting to fill metal cutting needs by any practical way possible, and is a descendant of electropolishing.
Electrolytic cutting, shaping, drilling, etc., without working the surface and leaving no chips or burrs, directly provides a product without surface distortion of any kind. All such products are directly inspectable for 100 per cent examination as to metallurgical quality, and are ready for the simplest array of finishing steps thereafter. If a worked surface is wanted, it can be attained by mechanical treatment to accomplish that work.
In conclusion, I leave with you the following thoughts. The members of the AES have skills being used to only a part of their actual worth. We are challenged to put those skills to more effective and extensive use.
Apply imagination. That is to say, visualize things as they can be, by using what we have now, but are not using. Keep research several steps ahead of what we need. Keep it oriented toward predicting what must be next, instead of staying behind because of explaining what has already happened.
A challenge is to face the discipline of providing and using continuous, on-the-spot controls for process and for product properties; and, provide the self-policing necessary. This can be done by research on ways to utilize electrochemical and electronic principles for the instrumentation we should have. We must make fullest use of skills, technology and science outside our own fields toward improving our processes and products.
Electrodeposition from fused salts and organic electrolytes must not be overlooked. Water solutions are certainly easier to work with, and have received most of our attention. Some improvements will come by advances of small degree. But, truly big gains will come from using new media and new combinations of methods; electrodeposition with vacuum deposition, vapor plating, plasma jet deposition, etc. Our challenge is to face up to using the technologies around us to the hilt. If we do not, some other group will.
We must develop a more professional status. A finishing and electrodeposition engineer must sit with design, materials and production (or products) engineers at the planning stage.
Finally, we must join with other societies in controlling the nature of publications we generate so that they are truly informative.
About Dr. Charles L. Faust (from the biography printed in 1960 at the time of his receiving the AES Scientific Achievement Award)

He is a member of The American Chemical Society, The Electrochemical Society, The American Electroplaters' Society, The Institute of Metal Finishing, and is a charter member of the Columbus Branch, AES, for which he has served on the board of managers and as a Delegate. He was co-recipient of the Proctor Award in 1940, of AES gold medals in 1951 and 1955, of the Carl Heussner award in 1955 and winner of the AES Scientific Achievement Award in 1960.
He was an associate editor of the Monthly Review and Plating, publications of the AES from 1942 until 1949; associate editor of Modern Electroplating, second edition, 1953; associate editor of Electroplating Engineering Handbook, 1955. At the present time, he is section editor of Chemical Abstracts, Section 4, Electrochemistry.
Dr. Faust was a member of the board of directors of the Electrochemical Society from 1943 to 1945 and 1947 to 1951. He was a vice president from 1947 through 1949 and was president of the Society in 1950.
During the war, he served on a subcommittee of the War Metallurgy Committee and was associated with special research and development for the war effort. Now he is a consultant for the Materials Advisory Board of the National Academy of Science. He is a registered Professional Chemical Engineer of the State of Ohio.


















