MicroCare's Stereze Product Line Receives FDA Registration
MicroCare has received FDA registration for its Stereze Hand Sanitizer line, which encompasses a spray, a gel and wipes.
Edited by Evan Doran
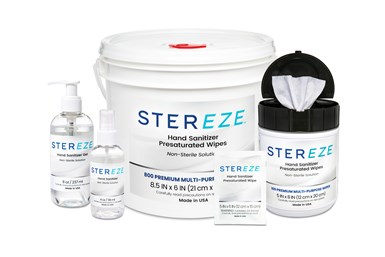
MicroCare LLC’s Stereze Hand Sanitizer Wipes, Gel and Spray have received registration from the U.S. Food and Drug Administration (FDA). MicroCare launched these sanitizers in late 2020 to support hand hygiene at work and at home.
“We are delighted to have received formal confirmation from the US FDA for the registration of our product monographs for Stereze Hand Sanitizers,” says Emily Peck, MicroCare senior chemist. “Since launching the products in November 2020, we have received high demand from businesses looking to keep their employees safe and enhance hand hygiene practices within the workplace. FDA registration is an important step for MicroCare LLC and will give customers the added confidence that Stereze Hand Sanitizers are safe, have been produced to the highest standards and are a highly-effective solution for hand sanitization.”
FDA-registered Stereze Hand Sanitizers are available in multiple form factors, with spray, gel and wipe options. MicroCare produces all forms of the Stereze Hand Sanitizer using a high-purity alcohol-based fluid.
RELATED CONTENT
-
Stripping of Plated Finishes
The processes, chemicals and equipment, plus control and troubleshooting.
-
Top Reasons to Switch to a Better Cleaning Fluid
Venesia Hurtubise from MicroCare says switching to the new modern cleaning fluids will have a positive impact on your cleaning process.
-
Cleaning Limescale from Galvanized Steel
How do you clean white lime scale and rust spots on galvanize?
















