Alumina Reinforced ENiP Offers a Versatile Substitute for Electroplated Hard Chromium
This study investigated the addition of Cirrus Alumina Dopant™, a nanoparticle additive, to a variety of low phosphorus electroless nickel baths to evaluate the performance of the resulting nanocomposite coating as a potential replacement for hard chromium. The results offer a versatile substitute for hard chromium in a broad range of applications.
#nasf
by
See Leng Tay, Pratik Jadhav and Chris Goode*
Cirrus Materials Science Ltd.
Auckland, New Zealand
Editor’s Note: A printable PDF version of this paper is available by clicking HERE.
ABSTRACT
The intrinsic hardness, high abrasive wear resistance, and corrosion properties of hard chromium coatings have resulted in their wide industrial application. However, chromium plating involves hazards associated with Cr(VI), which affect human health and drive the need to identify viable alternatives. This study investigated the addition of Cirrus Alumina Dopant™, a nanoparticle additive, to a variety of low phosphorus electroless nickel baths to evaluate the performance of the resulting nanocomposite coating as a potential replacement for hard chromium. A comprehensive comparison for the performance of coatings for alumina-doped electroless nickel and a pure low electroless nickel coating were investigated. Results showed that Al-doped electroless nickel possessed a minimum hardness of 850 HV0.1, high corrosion resistance, excellent abrasive wear resistance, and a Taber Wear Index of 2.25 mg/1000 cycles. These attributes suggest Cirrus Dopant™ for electroless nickel may offer a versatile substitute for hard chromium coatings in a broad range of applications.
Featured Content
1. Introduction
Hard chromium coatings are specified for the protection of high strength steel substrates for a variety of applications including aircraft landing gear, hydraulic rams, gas turbine engines, propeller hubs, etc., due to desirable hardness, abrasive wear resistance and other surface qualities. Due to both environmental and health concerns, regulatory action such as the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACh) in Europe and the Environmental Protection Agency (EPA) and Occupational Safety and Health Administration (OSHA) in the USA have limited the use of hexavalent chromium.1 A range of alternative coatings has been developed by diverse suppliers. However, for many applications a suitable replacement for hard chromium has yet to be identified.
Incorporating a variety of hard particles into plating baths, including Cr3C2, SiC, Al2O3 and nano-diamond to create composite coatings has enabled nanocomposite coatings to achieve the desired hardness and abrasive wear resistance.2 Unfortunately, very high particle concentrations have been required to achieve good abrasive wear, with an increase in the cost of chemicals. Incorporating a large volume of hard particles often produces excessively rough coating surfaces. This problem may be overcome by using a stable aqueous suspension of appropriate nanoparticles in the bath. Cirrus Dopant™ is an aqueous additive comprising a nano-structured precursor material that creates in-situ nanoparticles during the plating process. In contrast to hard particle methods, incorporation of Cirrus Dopant™ does not create any rough surface finishing, and nanoparticles will deposit uniformly into the coating. Only a small volume of Cirrus Dopant™ is required to obtain the desired abrasive wear performance.
Nickel and its alloy coatings have been long identified as possible replacements for hard chromium, but these coatings suffer shortcomings, especially in abrasive wear performance.3 The present work is focused on incorporation of Cirrus Dopant™ into low phosphorous electroless nickel (LPEN). Electroless plating is an autocatalytic coating process based on the chemical reaction between the plating solution and the substrate and compared to electroplating, can produce a homogenous thick coating on a complex geometric workpiece. This study reports the improved mechanical properties of low phosphorous electroless nickel (LPEN) coatings by introducing low proportions of alumina dopant (Al-doped LPEN). The performance of this Al-doped LPEN composite coating is also evaluated.
2. Experimental
Low phosphorous electroless nickel (LPEN) and Al-doped LPEN were deposited on mild steel. Before electroless plating, the specimens were thoroughly degreased and rinsed with deionized water. The nickel and hypophosphite content were controlled between 5.8-6.0 g/L and 18-22 g/L, respectively. The pH of the plating solution was maintained between 6.25 and 7.0, and the temperature was 86-90°C during plating. The effects of two accelerators and various thiourea levels were investigated. Standard industry titration methods were performed during plating to maintain the composition of plating bath. The plating bath lifetime was controlled to a maximum of four Metal Turn Overs (MTO). Hydrogen Embrittlement (HE)-baking was performed on all coatings, both doped and undoped, for 4 hours at 210°C.
The microhardness of the coating surface was measured using a load of 100 gf with a holding time of 10 sec. The reported results have been averaged across ten measurements. The wear testing was measured by rotating disc at 100 rpm under 2N with alumina ball as counter materials. Taber wear measurements were performed to evaluate the abrasive wear properties of coating. Two CS-17 abrasive wheels were placed on the specimen with a 1000 g load on each wheel and a sliding wear speed at 72 rpm. After each set of 1000 cycles, the specimen was ultrasonically cleaned in deionized water for 5 minutes before the weight loss was measured using a 0.1 mg resolution scale. Three mass readings were averaged to determine mass loss. The surfaces of the abrasive wheels were refaced using 150 grit size SiC paper to avoid clogging or contamination with wear particles after every 1000 cycles. The experiment was repeated ten times to determine an average of Taber Wear Index (TWI).
Potentiodynamic curves of LPEN, Al-doped LPEN coatings and uncoated mild steel substrates were measured by CHI650E electrochemical workstation. The tests were performed at room temperature, using a classical three-electrode cell composed of the working electrode with an exposed area of 1 cm2, saturated calomel electrode (SCE) and a platinum as the counter electrode in 3.5 wt% NaCl solution. Tafel plots were obtained at a scan velocity of 0.5 V/sec.
The phase structure of the coatings was characterized by Ultima IV, Rigaku X-ray diffractometer (XRD). Diffraction patterns were recorded in the 2θ range from 20° to 80° with a scanning step of 0.02°. The cross-section microstructure of the coating was analysed using a FEI Quanta 200 field emission environmental scanning electron microscope.
Adhesion of the coating was tested by bending the coated sample with pliers and observing the coating under an optical microscope. A ferroxyl porosity test for iron base substrates was performed based on ASTM B733. Here, the coated specimen was immersed in the test solution comprising 25 g/L potassium ferricyanide and 15 g/L of sodium chloride for 30 sec, then the specimen was removed, cleaned, air dried and examined for rust spots which appear as blue pores under an optical microscope.
3. Results and discussion
Figure 1 compares the microhardness and Taber Wear Index (TWI) measurements for both LPEN and Al-doped LPEN post HE bake. The incorporation of Cirrus Dopant™ in the LPEN bath increased the coating hardness from 597 to 862 HV. This hardness is much greater than that of common hard chromium, about 850HV.4 This improvement in hardness was mainly due to dispersion strengthening from alumina particles in the Ni-P deposit. Alumina nanoparticles dispersed uniformly in the Ni-P coating which hindered dislocation movement, and thus further increased the hardness.5
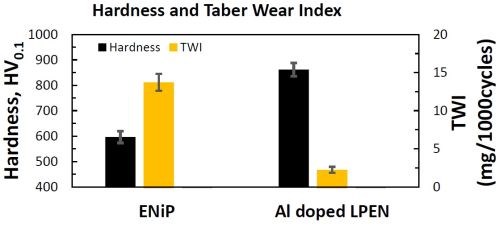
Figure 1 - Comparison of the hardness of LPEN and Al-doped LPEN.
The TWI of Al-doped LPEN was only 2.25 mg/1000 cycles, which is lower than the reported 3-4 mg/1000 cycles for a hard chromium coating.6 The lower TWI could be attributed to the hardness improvement created by Al incorporation and its dispersion strengthening effect in the Ni-P matrix.7 The measured TWI of Al-doped LPEN might also directly correlate with the coefficient of friction (COF) value.
Figure 2 shows the evolution of the COF between the two coatings. Clearly, the Al-doped LPEN has a smoother, more wear-resistant surface, which suggests a more compact coating.
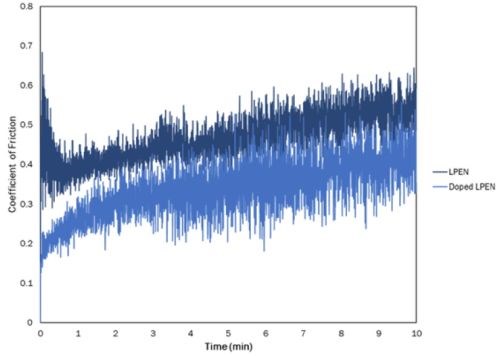
Figure 2 - Comparison of COF of LPEN and Al-doped LPEN.
Figure 3 shows an SEM image of the coating cross-section microstructure together with an EDS composition analysis. The phosphorus content from the EDS analysis was ~2.86 wt%, confirming that the coating was low phosphorus electroless nickel. The coating exhibits good adhesion to the substrate and no defects or cracks were observed at the interface. This indicates that the low internal stress deposit for a coating that has thickness for about 100 μm. The Al-doped LPEN coating showed a homogeneous structure without any visible alumina particles, evidence of their extremely small size and even dispersion. By comparison, phosphorus content measurements made on the same sample using Niton XL3 GOLDD+ XRF analyser reported approximately 1 wt%.
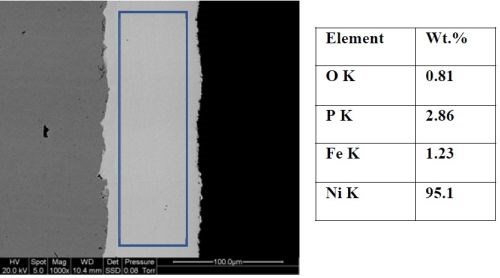
Figure 3 - Cross section SEM Image and composition of coating
The factors which lead to the improvement in the TWI were examined in a short study. Here total dopant additions and coating phosphorus content were analysed with the results shown in Figure 4. The primary contributor to TWI performance appears to be the phosphorus content of the coating (red dots) which was related to the total dopant added in mL/g of nickel added to the bath.
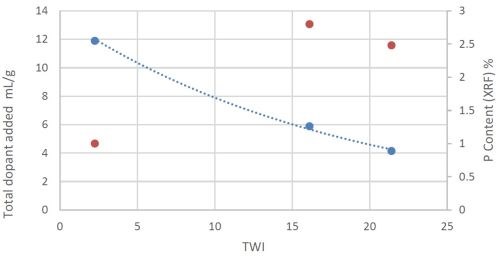
Figure 4 - Effect of phosphorus content and dopant addition rate to TWI.
The effect of bath composition and operation on the phosphorus content of the coating were examined. Three accelerators, various thiourea concentrations and a variety of operating conditions, as shown in Table 1, were evaluated. The accelerator chemical and thiourea additive had a significant effect on the phosphorus content. Operating temperature and sodium hypophosphate concentration mostly affected the plating rate. Interestingly, the coating hardness measured was little affected, suggesting that the dopant operation was consistent within experimental error over the range of bath operations examined.
Table 1 - Effects of bath composition and operation on phosphorus content.
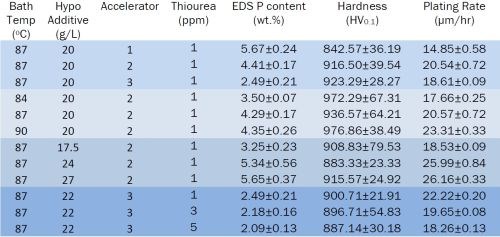
Figure 5 shows the comparative Tafel plots for the three coatings. The addition of alumina into the Ni–P matrix reduced the corrosion current density and increased the positive corrosion potential, indicating higher corrosion resistance than for simple Ni–P coatings.8 Data derived from these curves were used to calculate, the corrosion potential, the corrosion current densities, and the corrosion rates. Incorporating dopant reduced the corrosion rate to 0.0243 mm/year, a 16% improvement. This increase in the corrosion resistance could be due to the more compact coating produced and Al nanoparticles acting as a corrosion insulator.9
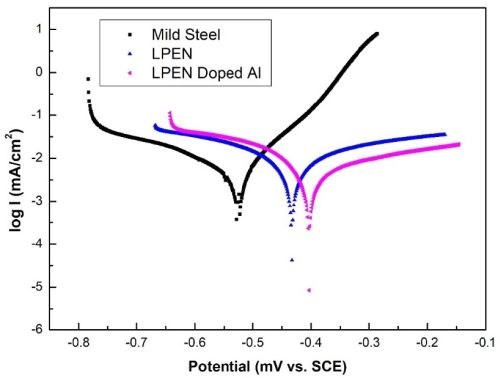
Figure 5 - Polarization curves of LPEN, Al-doped LPEN coating and mild steel substrate.
A Bending Test is the common method to evaluate the adhesion of the coating. In this research, the test method was more severe than a standard mandrel bending process, as the bend shape was unconstrained. The samples were examined under a microscope before and after bending as shown in Fig. 6. No peeling or flaking of the coating was observed. Minor cracks were observed under the microscope for both coatings. The good adhesion of the coating on mild steel plays a crucial role in the corrosion resistance as it protects the substrate from being attacked.
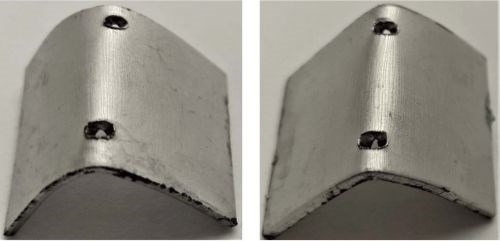
Figure 6 - Images of Cirrus LPEN (left) and LPEN (right) post bending.
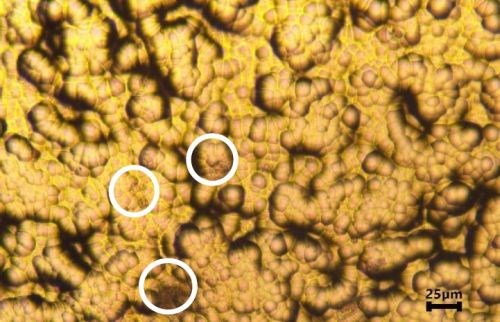
The optical images of Al-doped LPEN and LPEN after a ferroxyl test are shown in Fig. 7. A few small pores were observed for the LPEN specimen, as indicated by the white circles. However, no detectable pores were found on the Al-doped LPEN coated specimens. The porosity of electroless nickel coatings depends not only upon their surface roughness but also on their surface morphology.10 As mentioned earlier, incorporation of dopant into LPEN produced a more compact coating due to the dispersed secondary particles. The compact coating protected the steel substrate and prevented exposure during the ferroxyl test.
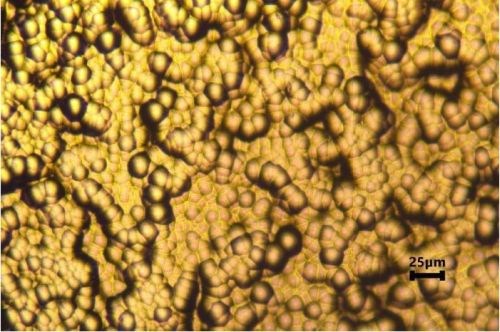
(a)
(b)
Figure 7 - Optical images of Cirrus LPEN (a) and LPEN (b) after porosity testing.
Figure 8 shows the XRD spectra for as-prepared, HE-baked and heat-treated specimens. All samples exhibited two broad peaks at ~44.5° and ~52°, which represent Ni (111) and Ni (200), respectively. No intermetallic peaks were observed after HE-bake, attributable to the low processing temperature. On the other hand, the specimen heated at 400°C for 1 hr, showed secondary intermetallic Ni3P peaks. Here, the X-ray diffraction patterns were sharpened, because all amorphous phases reverted to crystalline Ni and crystalline Ni3P at the temperature above 300°C.11 No Al2O3 peaks could be detected in the coatings, probably due to the low quantity of Al2O3 and high intensity of the Ni diffraction peaks. Less than 1 vol% of Cirrus Dopant™ was added to the bath solution with prototype particles in the range from 10-60 nm.
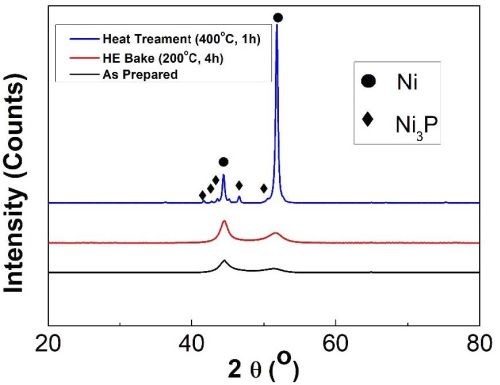
Figure 8 - XRD spectra of Al-doped LPEN for as-prepared, post-HE bake and post-heat treatment at 400°C.
The typical industrial post-treatment to harden an electroless nickel coating is heat treatment at 400°C for 1 hr to form intermetallic phases. However, this heat treatment and resultant intermetallic hard particle formation can shrink the Ni-P matrix, causing crack formation and reducing the anti-corrosion properties of the coating.12 Thus, in this research the coating was only baked at 200°C for 4 hr to eliminate hydrogen embrittlement caused by the plating without formation any secondary phases.
4. Conclusions
A high deposition rate, low electroless phosphorus nickel (LPEN) was successfully deposited on mild steel substrates. Incorporation of Cirrus Dopant™ as an additive in the LPEN bath significantly improved the mechanical properties of the low phosphorus electroless nickel coating. The microhardness was improved by 40% over LPEN and the Taber Wear Index of 2.25 mg/1000 cycles was less than that of hard chromium. Low phosphorus content was essential to achieving the Taber Wear Index. Bath composition and dopant replenishment rate contributed to achieving low phosphorus. The Al-doped LPEN also showed better corrosion resistance, good adhesion and no porosity. These desirable properties of Al-doped LPEN demonstrated its potential as a versatile substitute for hard chromium, contributing to the elimination of the use of toxic chemicals.
5. Acknowledgment
The authors gratefully acknowledge Ijya Srivastava and Jon McCrea for helping us to carry out Taber wear testing.
6. References
1. B. Podgornik, et al., “Crack density and tribological performance of hard-chrome coatings,” Tribol. Int., 121, 333-340 (2018).
2. G.A. Lausmann, “Electrolytically deposited hard chrome,” Surf. Coat. Tech., 86-87 (2), 814-810 (1996).
3. R. Bernasconi, et al., “Codeposition of nickel-phosphorus alloys reinforced with boron carbide microparticles: direct and pulse plating,” Trans. IMF, 95 (1), 52-59 (2017).
4. M.L. Klingenberg, E.W. Brooman and T.A. Naguy, “Nano-particle Composite Plating as an Alternative to Hard Chromium and Nickel Coatings,” Plat. and Surf. Fin., 92 (4), 42-48 (2005).
5. A. Hadipour, S.M. Monirvaghefi and M.E. Bahrololoom, “Electroless deposition of graded Ni–P coatings,” Surf. Eng. 31 (6), 399-405 (2015).
6. J. Gao et al., “Crystallization behaviour of nanometer-sized Al2O3 composite coatings prepared by electroless deposition,” Mater. Lett., 59 (2-3), 391-394 (2005).
7. P. Gadhari, S. Prasanta, “Electroless nickel-phosphorus composite coatings: A Review,” Int. J. Manuf. Mater. Mech. Eng., 6 (1), 14-50 (2016).
8. P. Makkar, R.C. Agarwala and V. Agarwala, “Wear and corrosion characteristics of alumina dispersed Ni–P nanocomposite coating developed by electroless technique,” J. Mater. Sci., 50 (7), 2813-2823 (2015).
9. A.C. Ciubotariu, et al., “Electrochemical impedance spectroscopy and corrosion behavior of Al2O3-Ni nano composite coatings,” Electrochim. Acta., 53 (13), 4557-4563 (2008).
10. P. Ernst, I.P, Wadsworth and G.W. Marshall, “Porosity of electroless nickel coatings investigated using different porosity tests and their application,” Trans. IMF., 75 (5), 194-198 (1997).
11. A.W. Goldenstein, et al., “Structure of chemically deposited nickel, J. Electrochem. Soc., 104 (2), 104-110 (1957).
12. T.S.N. Sankara Narayanan, et al., “Electroless nanocomposite coatings: Synthesis, Characteristics, and applications,” in: M. Aliokfhazraei, A.S.H. Makhlouf (Eds.), Handbook of Nanochemistry, Springer International Publishing, Switzerland, 2016; pp. 389-416.
About the authors
.jpg;maxWidth=600)
Dr. See Leng Tay is a Senior Materials Engineer at Cirrus Materials Science Ltd. in Auckland, New Zealand. She completed her PhD in 2016 at the University of Auckland, New Zealand under the “Bright Sparks” University of Malaya Overseas Scholarship. Her research areas include surface science and engineering, nanocomposite coatings, intermetallic compounds, lead-free solder, and she has published more than 30 journal articles and 2 patents. See Leng Tay has won awards at national and international exhibitions for her research work, including Gold medal in International and Technology Exhibition and Sparks Ideas Challenge 2015 (Commercial Ideas).

Pratik Jadhav is a Junior Materials Engineer at Cirrus Materials Science Ltd. He completed his Research Masters in Materials Engineering in 2017 at the University of Auckland. His research areas include nanotechnology-enabled welding wires, surface science and engineering and antiviral coatings. He received the NZ Aluminum Smelters Best Master’s Student award in 2017, pertaining to his research work and publication during his Masters.

Chris Goode is a founder and Chief Technical Officer at Cirrus Materials Science Ltd., in Auckland, New Zealand. Chris holds an honors degree in engineering from the University of Western Australia. He holds over 30 patents and has extensive experience in multiple fields of engineering including telecommunications robotics and chemical engineering.
* Corresponding Author:
Chris Goode, CTO
Cirrus Materials Science Limited
5B Target Court, Auckland 0627, New Zealand
Phone: +64 21 248 8853
Mobile: +64 21 248 8853
E-Mail: chris.goode@cirrusmaterials.com
RELATED CONTENT
-
Plastics and Plating on Plastics [1944]
This republished 1944 AES convention paper presents an historic perspective of the early days of plastics in surface finishing - using them and plating on them, in the waning years of World War II. The discussion reviews the uses of plastics in plating equipment and processing at that time, as well as the coating of the plastics themselves, with accompanying application photos. You will note that today’s conventional plating-on-plastics processes lay far in the future. Surprisingly, CVD processes are discussed.
-
A Process for Alkaline Non-cyanide Silver Plating for Direct Plating on Copper, Copper Alloys and Nickel Without a Silver Strike Bath
Traditionally, silver is electroplated in toxic, cyanide-based chemistry. Due to cyanide’s extreme hazard to human health and environments, developing non-cyanide silver chemistry is essential for the silver electroplating industry. Discussed here is an aqueous, alkaline non-cyanide silver plating technology, which can be directly plated over nickel as well as copper and its alloys. The silver deposits have perfect white color and better anti-tarnishing properties than other non-cyanide silver processes. The silver is plated entirely from the dissolving silver anode and the bath is very stable, and maintains a stable pH level both during plating and idle time. This new non-cyanide silver technology will plate bright silver that is perfectly suitable for electronic, industrial and decorative applications. .
-
Federal Courts Stay Implementation of OSHA COVID-19 Rules
In response to a backlash of litigation, multiple federal courts have blocked implementation of OSHA and other new vaccine mandates for general industry, the healthcare industry and federal contractors.


















