Plastics and Plating on Plastics [1944]
This republished 1944 AES convention paper presents an historic perspective of the early days of plastics in surface finishing - using them and plating on them, in the waning years of World War II. The discussion reviews the uses of plastics in plating equipment and processing at that time, as well as the coating of the plastics themselves, with accompanying application photos. You will note that today’s conventional plating-on-plastics processes lay far in the future. Surprisingly, CVD processes are discussed.
#nasf #masking #vacuum-vapor
by
Harold Narcus
Plating Processes Corporation
Holyoke, Massachusetts, USA
Editor’s Note: Originally presented at the Educational Sessions of the 32nd AES National Convention in Cleveland, Ohio [H. Narcus, Proc. AES, 32, 76-92 (1944)], this paper presents an historic perspective of the early days of plastics in surface finishing — using them and plating on them. At the time, plating on non-conductors was coming into its own, spawned by the emergency of World War II. The D-Day invasion had occurred just a week prior to the Convention (June 12-14, 1944). Though much progress has been made in the intervening 77 years, it is worthwhile to explore what were the early perspectives in this surface finishing field. The discussion reviews the uses of plastics in plating equipment and processing at that time, as well as the plating of the plastics themselves, with accompanying application photos. You will note that today’s conventional processes lay far in the future. Surprisingly, CVD processes are discussed. A printable pdf of this paper can be accessed and printed HERE.
Featured Content
Introduction
Although the war is still in its most critical stage, postwar planning has begun to occupy the minds of the leading manufacturers in the country. The electroplater, when this conflict eventually terminates, will be confronted with many new materials and processes. Plastics, for example, have become important materials in industry and will find innumerable additional applications. The process of plating on these plastic materials is presently aiding the war effort tremendously. The ultimate result will be a very close relationship between the plastics and electroplating industries. Therefore, the material in the following paper has been compiled with one purpose in view, namely, to present the electroplater with an insight into the field of plastics and plating on plastics which today is playing such an increasingly important role in his expanding industry and which will, in the future, gain in popularity.
The plater is continually confronted with the problem of masking parts for localized deposition as in salvage work or in preparing parts for selective hardening. In addition, it is frequently necessary for him to stop-off racks for plating to increase their life and efficiency besides for conservation of critical metals needlessly deposited on these racks. Proper linings for plating tanks is also commonly considered his responsibility. Furthermore, he is faced with the task of plating on plastic materials, a process used for relieving the acute shortage of base metals and for various functional purposes. This procedure is definitely increasing in importance but is also presenting the plater with many new problems.
With this in mind, the speaker would like to attempt to clarify many confused ideas regarding plastics and the plastics industry including plating on plastics which the plater may possess without probing too deeply into the field of organic chemistry. It must be remembered that the field of plastics is a tremendous industry with new developments occurring daily and an industry about which volumes could be written. It is hoped, however, that the presentation of this paper will give those assembled here an impetus to investigate a new series of organic materials, an employment of which will produce better work more rapidly and economically with an increase in quality, and to utilize the process of metallization of plastics wherever possible.
1. Organic plastics
Organic plastics, in most cases correctly termed "synthetic resins," are moldable materials manufactured from organic compounds which are combinations of carbon with nitrogen, oxygen, hydrogen and other elements. These plastics are members of that vast group of materials which are readily responsive to shaping influences, such as pressure in the presence of high temperatures - materials which maintain their new form when the shaping influences are ultimately removed.
The general types of organic plastics, in order of their importance to the plating industry, are as follows:
- Synthetic resins including the cellulose derivatives
- Natural resins
- Protein derivatives
The natural resins which include shellac, rosin and the asphalts find numerous applications in the plating room, but they are more familiar to the plater than the synthetic resins and therefore will not be discussed in this paper. The protein derivatives, the most distinctive member of which is casein, have found little use in the plating room to date and, hence, will also be omitted. Thus, the scope of this paper will include the newer yet far more important group, namely, the synthetic resins, including the cellulose derivatives and deposition on these resins.
Before discussing this group classification more specifically, for the sake of clarification, it would be advisable at this point to subdivide plastics still further as follows:
- Thermo-plastic resins
- Thermo-setting resins
The first group, that is the thermo-plastics, embodies those which are softened, molded or shaped by pressure, with the aid of heat or without it, and whose final hardened shape can be re-softened by heat or in other words, re-molded. The second group represents those which in their original condition, flow and can be molded by heat and pressure, but which once in their final state or so-called "cured" condition cannot be re-softened or re-molded. Familiar members of the former category of materials are the "vinyl" resins, "styrene" resins, "acrylic" resins cellulose compounds and the increasingly important synthetic rubbers. The thermo-setting resins include the phenol-formaldehyde compounds, urea-formaldehyde and melamine-formaldehyde resins.
The first previously mentioned general group of resins- -the synthetics - as previously stated, are perhaps the materials which are mostly used in the plating industry at the present time. In opening the discussion of these substances, let us begin with the synthetic resinous materials which are thermoplastic.
The resins which the speaker has found to be of utmost importance especially in the field of "hard" chromium plating are the "Vinylite" co-polymer resins. They possess extreme chemical inertness and are unaffected by alkalis, oxidizing agents and most inorganic acids. Water, alcohol, grease, fats or aliphatic hydrocarbons have no effect on them. They are, however, swelled by chlorinated hydrocarbons and other organic materials such as ketones (i.e. acetone, methyl isobutyl ketone, etc.) and aldehydes, besides certain organic acids. In sheet form, the "Vinylite" plastic is tough, highly resistant to tearing, abrasion and scuffing. It is elastic and can be stretched to 200-300% of its original length. Because of their low water absorption and their lack of physically incorporated plasticizers, the "Vinylite" co-polymer resins are dimensionally stable and will not warp or shrink on aging or under varying conditions. They may be shaped under heat as desired. This property is very advantageous in utilizing this material for masking as in "hard" chromium and heavy nickel plating.
These co-polymer resins are also used for surface coating applications. The polyvinyl chloride solutions in particular, make up the basic ingredient for excellent "stop-off" lacquers in electroplating or for making metal surfaces chemically resistant. Of particular importance are thin coatings applied to iron to replace materials such as tin-plate, galvanized iron, aluminum and other strategic or critical supplies which no longer are readily available. When the polymer resinous substances are applied from solution in organic solvents, they yield a tough, glossy, chemically resistant coating upon baking. Because of their flexibility, toughness and adhesion, they can be used as finishes on sheet metal which is to be fabricated after being coated. Parts may be stamped, spun or drawn from the coated sheet without any cracking or rupturing of the film.
In the plating room, care must be taken not to subject the film or "Vinylite" sheet employed to high temperatures, as it has a relatively low softening point (around 160°F).
Vinylidene chloride resins have aroused considerable interest because of their unusual properties, namely, water, chemical and solvent resistance besides toughness and durability. It is suitable for many difficult industrial applications. In the plating room, this synthetic resin in tubing form is excellent for covering lead wires and metal tubing connections for thermal control instruments and for protecting other wires leading to voltmeters and ammeters, insuring the wires against the corrosive action of the electrolytes or especially the spray from gassing of parts therein as in chromium plating. Molded fittings made from this material replace those formerly made of copper and brass - metals which are most critical. It is also used for masking off plating racks and for making lacquers and corrosion-resistant tapes.
This resin has a relatively high softening point (around 240°-280°F), ease of machining, non-inflammability, high tensile strength, high fatigue and abrasion resistance and high dielectric properties.
The polyvinylidene chloride resin is known to the trade as "Saran." It can be obtained in practically all forms such as rods, tubing and sheets.
Other resinous products similar chemically to the "vinyl" resins just discussed by virtue of the presence of the so-called unsaturated vinyl radical (CH2 = CH-) in their molecules, are the "styrenes." (The primary "styrene" has the formula C6H5,CH = CH2.) These resins find use in various metal lacquers and light colored enamels. They dry smoothly without cracking and have a high luster. Their hardness and plasticity compare favorably with the phenol-formaldehyde resins and in massive form, they are somewhat stronger. When better synthetic methods of producing styrenes are available these resins probably will be of greater importance.
The polystyrene resin is a highly thermoplastic molding material with high insulating properties, moisture resistance, inertness, dimensional stability, because it contains no plasticizer, and possesses high impact strength. It is soluble in cheap solvents and coatings of polystyrene are quick-drying, resistant to water and moderately so to acids and alkalis employed in the plating room.
Because of its low specific gravity (1.04), cubes of polystyrene can be readily used to float on the surface of plating solutions which are not operated at too high a temperature, for keeping spray from entering the atmosphere of the plating-room as in the case of chromium baths or iron baths operated at moderate temperatures. Polystyrene should not be subjected to high temperatures (above 175°F) for too long a time as it will soften considerably under such conditions. This is true when it comes in contact with hot alkaline cleaners. Loalin, Lustron and Styron are well-known members of this group of thermo-plastic materials.
A relatively new type of resin, which first appeared on the market in 1935, and which attracted the attention of the optical industry because of its amazing optical properties is the "acrylic" resin. It is clearer than glass and has assumed great importance as an "organic" glass.
The polymethylmethacrylate resin, known to the industry as "Lucite," "Plexi Glass" and "Acryloid," is one of the most used of the acrylic resins. This substance has a high softening point and is capable of being immersed in boiling water for short periods without distortion. It is characterized by its elasticity and remarkable impact strength.
Coatings made from this resin have good insulating properties. They are used for hard heat-resistant enamels and for solvent-resistant finishes for metals. They are especially utilized in white enamels because they will not discolor when maintained for long periods of time at temperatures as high as 500°F and also in fume-resistant enamels. In general, they are compatible with nitrocellulose.
The polymethylmethacrylate resin has found excellent use in the plating room on safety goggles and hoods when handling corrosive liquids and during grinding and deburring operations.
Its resistance to both weak and strong alkalis and acids is excellent. It is highly resistant to mineral, vegetable and animal oils. "Lucite" is being used extensively for a rapid method of hard chromium plating aircraft parts such as landing gear pistons for dive-bombers. The rod or tubing of this material is usually machined to properly fit and shield the desired area. This plastic withstands the cleaning cycle solutions and chromium plating bath during the entire operations without evidence of deterioration. Unfortunately vapor degreasing is not possible when using this material.
Plastics derived from cellulose were among the first to be developed. The most important example, celluloid or "Pyroxolin," is made by compounding cellulose nitrate with camphor, but because of its very high inflammability, it has been partly replaced by other cellulose materials on which the fire risk is less great. However, it must be mentioned at this point, in fairness to the cellulose nitrate industry, that the cellulose nitrate plastics are among the oldest of the cellulose compounds currently manufactured and are most prominent in lacquers which are used in the synthetic finishing and plating industries.
A cellulose nitrate product which has found wide use in the electroplating industry and which warrants some discussion at this point is Plastic Wood. It is made by mixing cellulose nitrate with wood flour. The procedure in its manufacture is as follows: the cellulose nitrate and a plasticizing agent such as camphor are dissolved in a blend of solvents containing acetone and then mixed with finely ground sawdust. The acetone evaporates leaving a hard mass resembling wood. If too rapid drying is evident, toluene or any similar solvent possessing the same rate of evaporation may be used as a retarder. The resulting product is used very advantageously in salvage work necessitating the "stopping-off" of keyways, slots, splined-areas, center holes, etc. It is usually advisable to top this plastic wood with an ample coating of lacquer to insure against its disintegration in the plating bath. This is particularly necessary in the case of "hard" chromium plating.
At the present time, wherever possible, cellulose acetate plastics have replaced those plastics made from cellulose nitrate to a great extent. They belong to a large family of cellulose derivatives which also includes the butyrates and ethers of cellulose, the latter called ethyl cellulose. In the United States, they attribute their development to the enormous availability of the basic raw material, cellulose.
The cellulose acetate plastics are typical representatives of this thermoplastic group of materials. Mechanical strength and toughness are their outstanding characteristics. The present metal shortage has been relieved greatly by utilization of the cellulose acetate plastics, especially in the hardware industry. Even in peacetime, there are many hardware articles which will continue to use these plastics in their makeup. Some examples are doorknobs, escutcheons, draw-pulls, faucet-handles and scores of other items. The plater will in many cases be called upon to electroplate these necessary articles in order to obtain certain properties of the outer metal. Some trade names which represent cellulose acetate are Lumarith, Plastacele, Fiberloid and Tenite I.
Cellulose acetate butyrate resembles cellulose acetate closely, especially in properties, uses and even appearance. It has excellent dimensional stability and low water absorption. At the present time, decorative strips of aluminum, brass, copper, nickel and chromium plate have been displaced by extruded cellulose acetate butyrate in wall-board trim, counter nosing and edgings, weather stripping and in innumerable other applications.
It has fairly good resistance to all plating baths, even chromic acid electrolytes, if not subjected to the solution for too long a time or at too high a temperature. Tenite II and Tulox are cellulose acetate butyrates which are familiar names in the trade.
Another member of this category of materials is ethyl cellulose, which is widely used as a base material for plastics and especially coatings, because of its solubility in cheap solvents, and compatibility with a wide variety of modifying agents such as resins, plasticizers, oils and waxes. The coating has heat resistance in addition to good thermoplasticity, does not readily age, has low inflammability and possesses good electrical insulation properties.
Lacquers made from this material can be brushed, sprayed or dipped on the parts. As in the case of vinylidene chloride resins, previously mentioned, ethyl cellulose plastic fittings are being used to replace copper and brass fittings which are scarce and to decrease the weight of these parts and similar parts.
Among its outstanding properties are its resistance to extremely cold temperatures and water, its toughness and high impact strength and its dimensional stability. It is also resistant to both weak and strong alkalis. It possesses good electrical characteristics and ease of fabrication. Ethocel is a well-known trade name for this material available on the plastic market.
The very important group of thermoplastic materials familiar to all as the "Synthetic Rubbers" should be taken up at this point but time does not allow a proper discussion of these most important materials, which warrant a close investigation by the electroplating industry in the very near future especially, after the termination of the present conflict.
The next important main group of synthetic resins, which perhaps are more familiar to the electroplating field, are the thermosetting resins principally the phenol-formaldehyde types bearing the trade names Bakelite, Catalin. Micarta, etc. These are probably more utilized by the plater than those just discussed, but as time goes on, these resins are finding the newer ones replacing them in many applications. Still, there is no doubt that, perhaps the greatest single factor contributing to the rapid growth of the plastics industry was the introduction of the phenol-formaldehyde resin.
Molding, casting and laminating are the three general methods for obtaining products from this type of resin. For use in molding compositions, the resins are strengthened with fillers which not only reduce the cost, but also by proper choice furnish desirable qualities to the finished product. Wood flour, when properly incorporated, gives a resulting resin having suitable flow with less shrinkage and greater toughness. Finely divided asbestos is used when resistance to heat is essential. Powdered graphite filler is used for low friction products. Bakelite, Resinox and Durez are examples of phenolic resins of the molding type. These resins are resistant to the atmosphere and chemical corrosion, have fairly light weight, low heat conductivity, high structural strength and excellent electrical insulation. They stand up well in most plating baths and alkaline cleaners, specifically to a large degree in nickel baths, but only fairly well in the conventional chromium bath. This is especially true when the material is not subjected to the chromium bath for too long a time. It would be in order to remark that when using plastics as stop-offs or in rack construction, they are not subjected to as severe an attack as when they are used permanently in the electrolyte, as in tank construction parts. Time element is thus important when using these plastics in the plating room. This type of resin also has excellent resistance to ketones, esters and to aliphatic and aromatic hydrocarbons in addition to animal, vegetable and mineral oils.
In constructing racks where certain parts of the rack merely serve as supporting platforms for the work and there is no need for a current-carrying medium at that part of the fixture, the phenol-formaldehyde resin of this type is excellent because of its relative rigidity or non-compressibility.
Catalin is a member of the group of phenolics known as the "cast" type. This type of resin usually contains a larger percentage of formaldehyde than the preceding one and usually does not employ any fillers. The resin is poured in a syrupy state into molds. A slow baking causes a hardening of the mass. Catalin has about the same efficiency in the plating room as Bakelite when used for relatively short lengths of time in the corrosive electrolytes, especially chromic acid, but I have observed that it withstands the action of the chromic acid electrolyte to a somewhat greater degree.
When the phenolic resinous substances are used as binders for such materials as cloth or paper, a third type called laminated phenolics results. This type is hard, dense and possesses excellent mechanical and dielectric properties. It finds an excellent place in rack construction when the racks are used in electrolytes which are not too harsh to the material. Micarta is a representative of this type. It is used to a great extent for insulating the anode from the cathode in set-ups for "hard" chromium plating using auxiliary anode construction. It finds innumerable other uses in masking parts.
Urea-formaldehyde, thiourea-formaldehyde and melamine-formaldehyde resins are thermosetting plastics which are definitely increasing in importance and should be given close investigation regarding their utilization in the plating room. The urea-formaldehyde resin is probably best known because it is used in baking enamels which are among the few that show excellent adhesion to electro-deposited chromium.
Before concluding the treatise of these synthetic resins, I would like to merely mention a relatively new process which should be of great interest to the plater.
This process employs these formaldehyde resins in a most unique manner. These resins, called "Amberlites" in the trade, are used as synthetic cation and anion exchange resins in water treatment. By use of this method it is commercially possible to produce quantities of water free from soluble salts economically. Innumerable uses can be found for this process in the silver plating and precious metals fields.
2. Plating of plastics
In addition to the proper selection of stop-off materials for racks and parts, possibly the best example of a situation wherein the electroplater must have a fair knowledge of the field of plastics is the problem of electrodeposition on these organic base materials. This type of electroplating which imparts metallic properties through deposition of a coating of metal to the plastic is increasing tremendously in popularity.
At the present time the two main purposes for metallizing plastics are, firstly, to render the plastic a suitable substitute for critical and strategic metals and, secondly, to produce an article which has the inherent properties of plastics with the desired properties of those metals. The deposition of metallic coatings on plastics also allows the various manufacturers to use certain plastic materials in a particular product which ordinarily could not be utilized. For example, mixed scrap plastics of one certain type but of a plurality of colors can be molded into the desired shape of the product and then plated, thus making use of idle scrap. Furthermore, the undesirable properties of the plastic such as its absorption of oils, solvents and moisture is eliminated by the proper metallic deposit. Swelling or distortion of the base organic material is thus prevented. Plating increases the heat and impact resistance, dimensional stability of the plastics and, hence, the rigidity of the original plastic part is increased. Probably the most outstanding advantage of this type of plating is the greater corrosion resistance of a metal deposit when it is applied on a plastic material than on the usual metallic base. The reason for this is very evident. There is no electrolytic action between dissimilar metals and the troublesome "spotting-out" condition is greatly eliminated.
The metal plating of plastics has opened up a vast new field in electricity and electronics. Plastics, particularly, the styrenes and phenols, possess excellent electrical insulating qualities, as previously pointed out, so that the plating of these materials make available to the electrical and electronic industries a process combining all the inherent advantages of the plastics plus the required properties of electroplated metal.
Aluminum and magnesium are being replaced by plated plastics in aircraft for electrical and radio shielding devices. This procedure eliminates costly inserts and assembly operations and gives a product which is lighter, more quickly fabricated and much more resistant to vibration. Frequency modulation and television antenna when manufactured by this process are improved in their performance since the reduction in weight permits a larger diameter tube or rod to be used in the element, increasing surface area and consequently pick-up. Multi-element antennas made by this method facilitates their installation and a simpler supporting structure can be used.
Of the methods available for metallization of plastics there are five principal ones, each with its own particular advantages and faults. These methods are the following:
- The varnish-conducting powder method and those very similar to it, such as the uses of the wax-graphite or plumbago combination, lacquer-copper powder or shellac-silver sulfide method.
- Metal spraying method
- Cathode sputtering
- Metal evaporation
- Chemical reduction
The first method, using varnish-conducting-powder, is the oldest of all the methods and is still being used to some extent today. It produces excellent, adherent deposits but it is not very suitable for work done on a vast production basis because of the time element involved in the varnishing and powdering operations. An objection to this process is also the fact that uneven deposits result and where it is necessary to reproduce the fine detail of the plastic this method fails considerably.
The second method, that is the use of the metal spraying gun, is objectionable because unfortunately there is a tendency to produce a granular or "sandy" effect. In this process the part is first cleaned and sand-blasted to produce adequate anchorage, after which molten metal, usually a low-fusing alloy like lead-tin is atomized and sprayed through a heated air gun at the work. If the gun is held too close the heat developed may either burn or soften the plastic. If held too far away the sandy effect is exaggerated.
The cathode sputtering method, although gaining in prominence, is very costly and is difficult to use for practical production purposes. The plastic acts as anode in a vacuum chamber opposite the cathode made of the metal, such as gold, silver or aluminum to be sputtered. Under a high vacuum, a charge of 10,000 to 20,000 volts is put on the electrodes and molecules of metal are literally torn out of the metallic cathode and deposited on the plastic. The equipment needed is expensive and there is a wasteful use of the metal deposited. It is used in record making and in the manufacture of tinsel and gift wrapping paper. Silver coated cellophane is made by using this method.
Metal evaporation is similar to cathode sputtering. A vacuum chamber is used to house the plastic work and the metal to be evaporated is made in a filament form. This filament is heated to incandescence by an electric current. The evaporated metal condenses like steam on the cool plastic, thus coating it. This process is also expensive but is used to produce metallized rayon coated with aluminum.
The last method, namely the chemical reduction process, is in my opinion best adapted to a production setup, is more economical, gives more uniform results than those methods just mentioned and is an easier method to manipulate. There are innumerable methods described in both the trade journals and patent literature on this type of electrodeposition but generally they accomplish the same results using the same basic procedure - namely the application to the plastic surface, after proper preliminary treatment, of a highly conductive and strongly adherent bond coat usually using an ammoniacal silver solution and a suitable organic reducing agent, followed by an intermediate layer of copper and finally a top layer of the desired metal such as chromium, nickel, gold, silver, cadmium, zinc, etc.
However, at this point, I would like to remark that in order for the plater to deposit any metal on a plastic base material or any non-conductive substance successfully, he must be given the "case-history" of the material so that he may prepare the surface properly for metallizing and obtain the most adherent metallic coating possible. All plastic materials, for instance, do not receive the same preparatory treatment. Some methods of preparation may work efficiently on one type of plastic while on another they may be harmful to the resin, causing attack, excessive swelling or even disintegration. In other words, the actual process of plating differs with the kind of plastic used. The differences mainly lie in the preparatory operations such as roughening and cleaning and the reducing agent used. It therefore follows that some knowledge of plastics is necessary for the successful plating of these substances.
The majority of plastics can be bonded for plating by using an ammoniacal silver nitrate solution used in conjunction with the cane-sugar reagent (Brashear Formula) or using formaldehyde or Rochelle salts as the reducing agent. ln other words, the ordinary mirror-making formulas can be employed. I have found best results with the formaldehyde. As previously stated, the method of preparing and cleaning the part prior to bonding depends on the synthetic resin used.
For example, Catalin, urea resins, Celluloid, polystyrene and methyl methacrylate must be given a wet-tumbling usually using pumice and water or given a light de-polishing, while cellulose acetate must go through an additional special caustic priming operation before actual bonding. Rubber is first treated with benzol or acetone and casein plastics with 3-4% hydroquinone.
The reducing agents used also depend on the original plastic material; phenol-formaldehyde plastics can be treated with hydroquinone, pyrocatechin and acetone for a few minutes then after thorough drying, placed in silver nitrate solution, and heated to 80°C. Acrylic plastics are bonded by reduction with cane-sugar, nitric acid, alcohol and water in the presence of ammoniacal silver nitrate solution. Casein plastics are handled by using hydroquinone or p-amino phenol for reduction. Urea or thiourea formaldehyde resins utilize boiling hydroquinone for reduction. The cellulose acetates are best bonded using formaldehyde and silver nitrate.
Sometimes, however, by knowing the type of plastic used, short cut methods for bonding can be used. For example, Bakelite can be bonded by merely immersing the resin for 1/2 hour in a mixture of silver oxide, ammonia and water.
Copper films, like silver, can be deposited on plastics. Just as in the case of silvering methods, films of copper are formed by reduction with formaldehyde or hydrazine. The chloride or sulfate solution of copper is usually used. There are innumerable formulas employed to deposit copper films on plastics. Gold films can be formed from an aqueous solution of gold chloride by using invert sugar, alcohol, citric acid or formaldehyde. Lead films can be applied using acetate with thiourea as the reducing agent. Nickel films are deposited from nickel carbonyl decomposed at 150°C.
In order to present this group with a clearer picture of the method of plating on plastics than can be obtained by reading the literature on this important subject, I would like to describe a process for plating on a common plastic material such as cellulose acetate.
Let us assume that the parts are small plastic pieces which must economically be handled in bulk. These parts are first wet-tumbled using pumice and water or blasted with 220 grain aluminum oxide until the gloss is removed and a dull sandy finish appears on their surfaces. This operation (wet-tumbling) may take 1 to 5 hours depending on the shape of the pieces. After thorough rinsing and drying, these pieces are cleaned in a cold caustic solution (dilute) for a short period. The parts are held in an ordinary acid-dip pot during this and the next operation. After thorough rinsing, the parts are treated with a stannous chloride-hydrochloric acid solution (which is patented) or a ferrous sulfate, hydroquinone or pyrogallol solution for a short period and then thoroughly rinsed. The parts are then placed into an inclined rubber bonding barrel which has enough water in it to cover the volume of the pieces. This barrel has ribs on its wall to insure against mass rolling of the parts and it revolves about 4 RPM. The proper amount of ammoniacal silver solution is added and then the proper amount of the formaldehyde solution. The quantities used depend upon the square inch area of work. The bonding treatment requires about 1/2 hour - depending on the shape of the parts. It may be necessary to repeat the bonding operation. The freshly precipitated coating is capable of taking other coatings.
After a suitable silver coating is applied, as determined by testing with an ohmeter or using a flash-light bulb connected in series with two test prods and a dry cell, the parts are rinsed thoroughly and allowed to dry. A hot air oven is recommended if overnight drying is not possible. When the parts are dry they are placed into an acid-copper plating barrel rotating at about 6 RPM. Large pieces can be bonded in the same manner on wires or racks and plated in still tanks.
The amount of acid copper deposited usually varies from .001" to .01" in thickness depending on the type of piece being plated. The thinner the deposit, the better the adhesion. After plating the parts are usually bright dipped and tumbled if in bulk or polished in the case of large pieces. The final plate can be any desirable metal.
I would like to review, at this point, some of the uses to which industrial deposition of metals on various non-conducting surfaces have been applied. This will prove the vast scope of this type of electrodeposition.
- For gas and liquid proofing containers.
- Electrical fuses, contacts, high tension devices, condensers, etc.
- Industrial cams, instruments, machine-parts, reflectors, rollers.
- Mirrors and molds especially for electro-forming.
- Scores of novelty and costume jewelry pieces too numerous to mention.
- Radio parts - loop antennae, cabinets, coils, condensers, dials, grilles, knobs.
- Airplane parts - dashboard parts, escutcheon plates and instrument parts.
3. Conclusion
In conclusion, may I say with due modesty, that I hope the presentation of this paper will assist the plater in selecting the proper resins in carrying out his particular type of work and will enable him to choose some of the newer, less-known plastics to perform these operations more rapidly, efficiently and economically. I am of the opinion, shared by many others, that the plastic and electroplating fields are becoming more and more related to each other as time progresses. At least some knowledge of the former field is not only convenient but advantageous to the plating supervisor. There is no doubt that intelligent investigation of the properties of the various plastics might suggest new outlets for these most versatile materials. I also hope that my brief treatise covering the field of plating on these plastic materials has been helpful in obtaining a clearer picture of this specialized type of electrodeposition.
5. Bibliography
1. Plastics Catalogue (1944) Plastics Catalogue Corp., NYC.
2. “Making of Mirrors by the Deposition of Metal on Glass," Circular of the Bureau of Standards No. 389.
3. G.M. Kline, "Organic Plastics," Circular of the Bureau of Standards C411.
4. U.S. Patent No. 2,303,871.
5. U.S. Patent No. 2,010,805.
6. U.S. Patent No. 2,243,429.
7. S. Wein, "Metal Coatings on Non-Conducting Materials," Part I and Part II, Metal Finishing, 39-40, Dec 1941 and Jan 1942.
6. Application photos
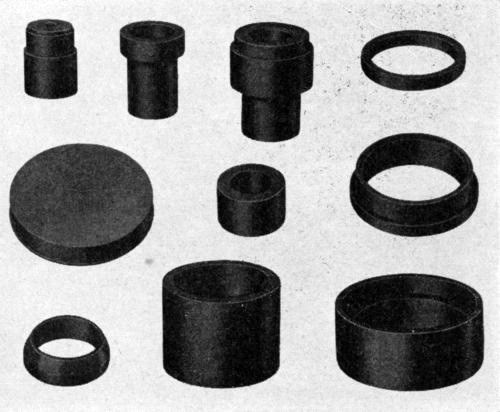
Figure 1 - Miscellaneous plastic shapes properly machined to fit a variety of machine parts for hard chromium plating. The shapes are inserted into or slipped onto the areas desired to be masked off.
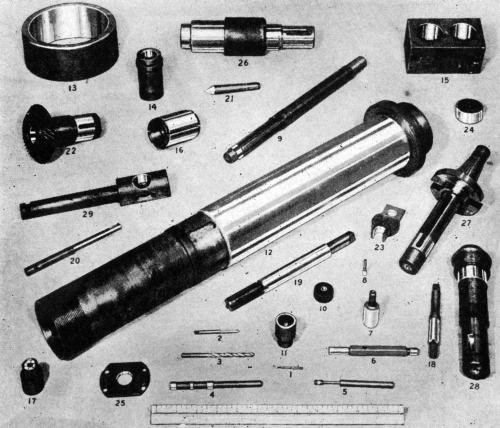
Figure 2 - Miscellaneous machine-parts and gages which were hard chromium plated on selected areas using plastic stop-off materials.
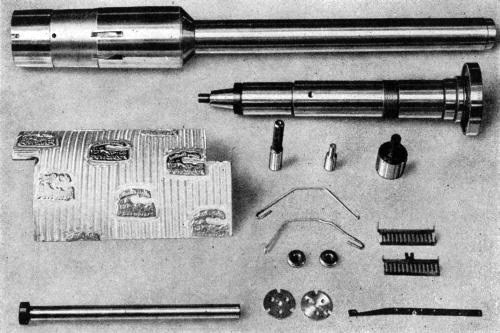
Figure 3 - Miscellaneous parts which were hard chromium plated on selected areas using plastic stop-off materials.
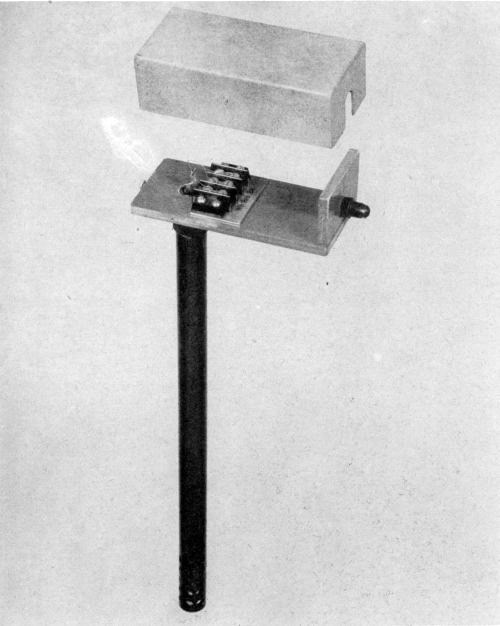
Figure 4 - Thermal responsive unit with plastic well and supporting platform
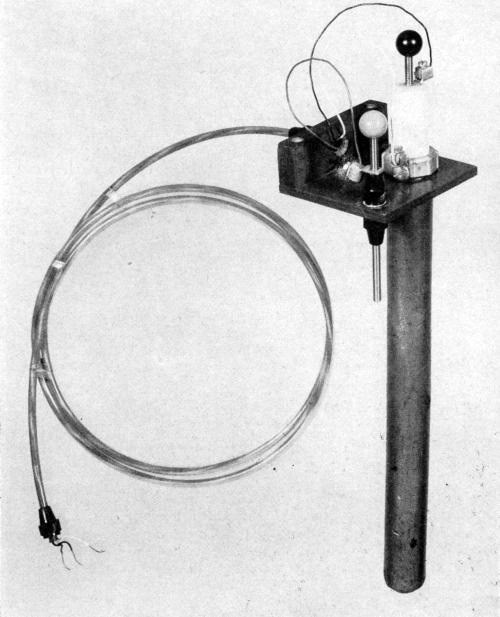
Figure 5 - Electronic pick-up unit with plastic fittings, platform and other component parts. Note the plastic covered lead wires and electrical wires to insure them against the corrosive action of the atmosphere prevalent in the plating room.

Figure 6 - Sensitive thermal control instrument with a protective plastic well to house the copper bulb. Note the plastic covered lead wires.

Figure 7 - A sump pump used in the hard chromium field for internal diameter plating. The pump is almost entirely made of plastic with the exception of the motor. The upright supports are plastic covered steel rods.
About the author
At the time of publication, Harold Narcus was Chief Chemist, Plating Processes Corporation, in Holyoke, Massachusetts, having worked there for the prior three years. He had is training at Polytechnic Institute (B.SD. Degree in Chemistry in 1934). He had spent the previous ten years in plastics and plating.
RELATED CONTENT
-
Qualitative Approach to Pulse Plating
In 1986, the AESF published Theory and Practice of Pulse Plating, edited by Jean Claude Puippe and Frank Leaman, the world’s first textbook on pulse plating. A compendium of chapters written by experts in this then-emerging field, the book quickly became the authoritative text in pulse plating. What follows here is the opening chapter, serving as an introduction to the field. Although the field has grown immensely in the intervening 35 years, the reader will find that the material remains a valuable introduction to those looking to advance the field of pulse plating.
-
Mechanical Properties of Electroformed Metals
In 1996, the AESF held its highly regarded electroforming course, prepared by Ron Parkinson for presentation by The Nickel Development Institute (NiDI) and the AESF. What follows is a slightly modified excerpt, specifically on the mechanical properties of electroformed metals. Much of this information has withstood the test of time, and gives a perspective of this technology at the turn-of-the-century.
-
Cyanide-Free Electroplating of Cu-Sn Alloys
This paper is a peer-reviewed and edited version of a presentation delivered at NASF SUR/FIN 2012 in Las Vegas, Nev., on June 13, 2012.


















