Published
Cr(III) Conversion Layers on Zinc and Zinc Alloys: Evolution and Perspectives
Trivalent chromium conversion layers have successfully replaced hexavalent conversion chromium on zinc and zinc alloys for many applications, especially in the automotive industry since the entry in force of the ELV directive.
#automotive
Lionel Thiery, Coventya S.A.Sm, Clichy, France
Introduction
Since the beginning of enforcement of the ELV,1 RoHS2 and WEEE3 directives, the world of conversion coatings on zinc and zinc alloys has changed considerably. The applicators for the automotive, electronics and building industries have turned to the use of Cr(III) conversion layers.
From the “basic” blue, thin conversion layer on acid chloride zinc to the thick nanoparticle-containing layer on alkaline Zn/Ni, providing outstanding corrosion resistance and a controlled coefficient of friction, continuous improvement has been undertaken in order to bring the conversion layers to the required high performance levels. These improvements have contributed to the high level of technical value added, such as in a fastener assembled in a car.
Besides the “psychological” issue of the color change from Cr(VI) to Cr(III), it has meant adopting the clear and black concept versus the yellow color, and primary concerns were related to achieving corrosion resistance performance equivalent to that of Cr(VI)-containing chromates, even after multiple physical and thermal shocks occurring in the life of a fastener part. Control over the coefficient of friction in contact with different materials was also a major requirement.
The desired performance was obtained thanks to the combination of Cr(III) passivates with top coat or post dip technologies. Despite these efforts to obtain outstanding substitutes for Cr(VI)-containing conversion solutions, newly issued European regulations such as REACH4 have led us to reinforce these developments.
Clear and black passivates
Clear conversion layers on zinc and zinc alloys were the first development of Cr(III) passivates with high corrosion resistance. Based on fluoride-containing solutions for creating thin layers, below 40 nm, and small layer weights (< 0.1 mg Cr/dm2), the thickness and layer weight has been increased by using trivalent chromium salts of appropriate type and concentration, adequate ligands for Cr(III), while increasing the temperature to favor Cr(III) oxide and hydroxide formation at the surface. As described by Barnes,5 nitrates are the oxidant of choice in these systems to initiate the zinc conversion mechanism.
The use of metallic inhibitors such as Co(II), Mo(VI), V(IV), contribute to the increase in corrosion resistance of the conversion layers.
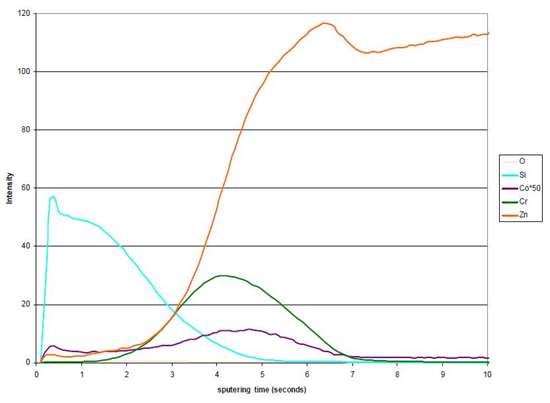
Recently, developments have been undertaken to incorporate amorphous silica particles in the conversion layers in order to reinforce the barrier effect of the layer and imparting better shock resistance to this non-self-healing coating. For specific parts, these systems have performed for more than 200 hr before white rust in the salt spray test according to ISO 9227.6
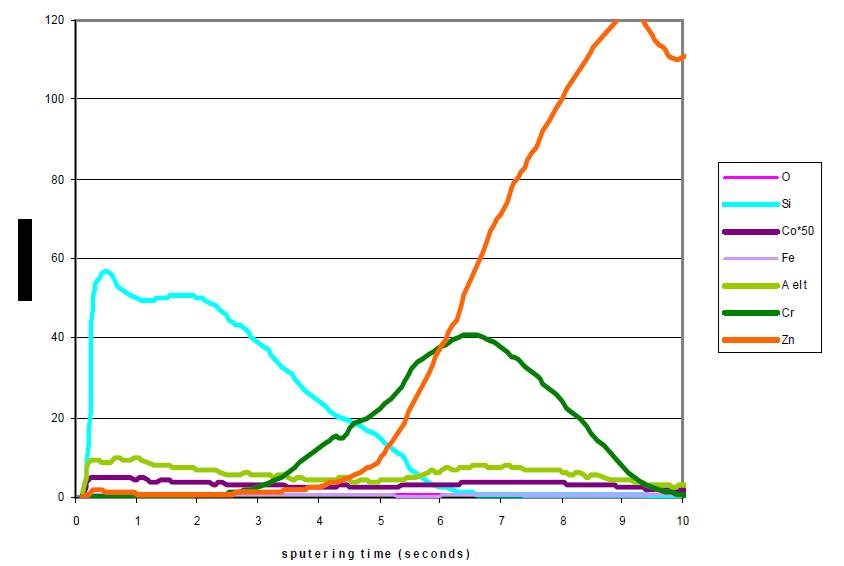
The barrier and improved shock resistance of the layer is evidenced by the glow discharge optical emission spectroscopy (GDOES) profile of a layer, where one can see the silicon profile on the outer side of the conversion film (Fig. 1).
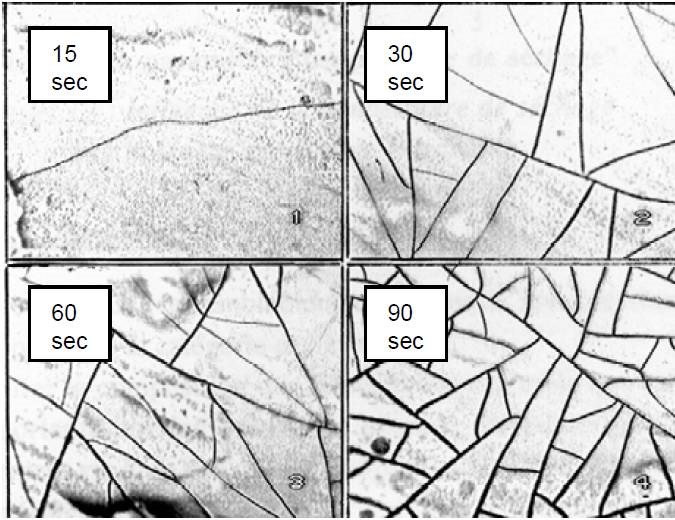
Black conversion layers were more difficult to develop, especially on pure zinc. On zinc alloys, the presence of an alloying element is often favorable to obtaining a black conversion coating. Zn/Fe in the presence of Cr(VI) leads to a nice black and bright appearance with a regular microcracked structure (Fig. 2).
Formation of a black conversion layer on pure zinc is more difficult since no alloying element is present to form a black oxide at the interface between the zinc deposit and the conversion layer. Additional elements are then incorporated into the conversion solution and codeposited in the conversion layer to produce a black oxide or sulfur compound. The morphology of the black conversion layer is not microcracked but quite porous as can be seen in Fig. 3.
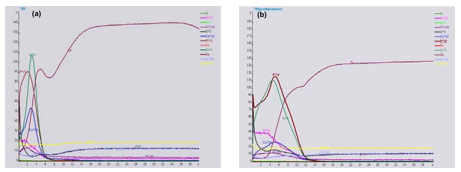
The layer thickness is also inferior to the thickness of a Cr(VI) based conversion layer, about 600 nm versus up to 2 µm for a black chromate on Zn/Fe. The corrosion resistance of this thin layer is then not in accordance with the automotive specifications which require up to 240 hr to white rust and 720 hr to red rust in neutral salt spray test after thermal shock, 24 hr at 120°C. It is necessary to add another layer on top of the black Cr(III) conversion layer. A post-dip based on trivalent chromium can nearly double the chromium layer weight of a Cr(III) conversion layer, as can be seen from GDOES profiles of a single black conversion layer and a layer covered with the post-dip (Fig. 4).
Post dips are of high interest for improving the overall aspect of a black conversion layer and enhancing the corrosion resistance but they don’t provide any control over the coefficient of friction.
Top coats
Top coats were developed in order to reinforce the corrosion resistance of chromates exposed to heat. A highly microcracked chromate layer would dehydrate during a thermal shock at 120°C and consequently show poor corrosion performance. The addition of a top coat layer using an organic binder and silica particles with high specific surface area provides good temperature resistance to the composite layer.
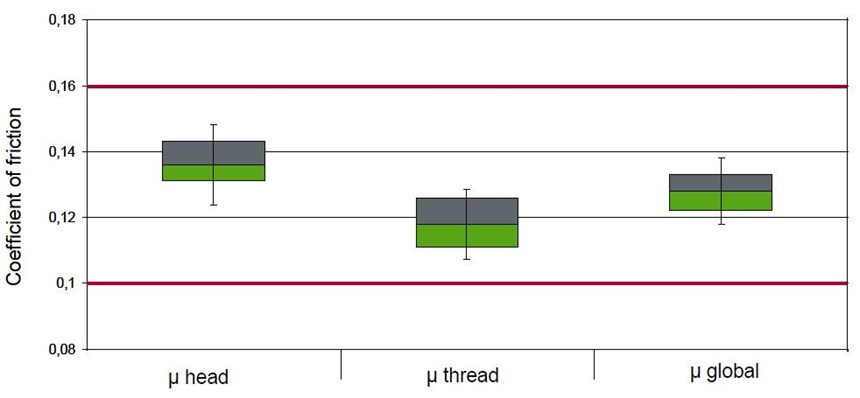
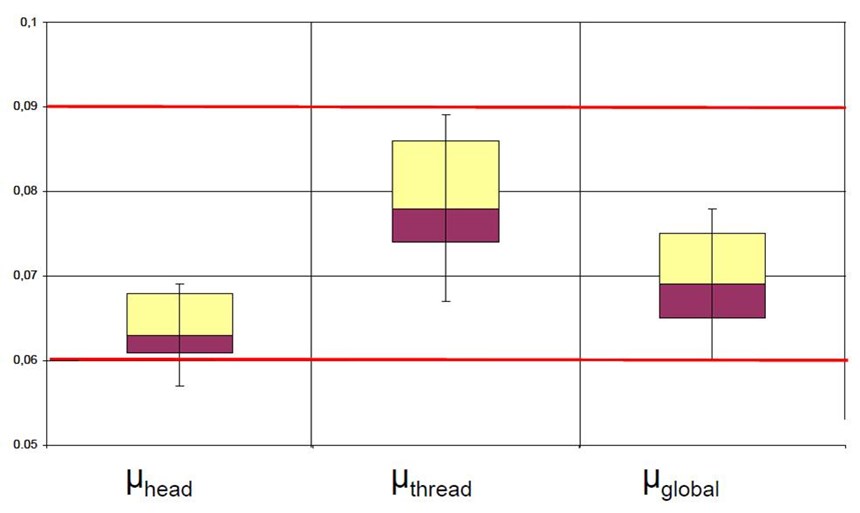
Additional requirements have to be fulfilled and in particular friction monitoring (torque-tension) in order to enable automatic assembly of screws on automobiles. Control over the coefficient of friction has thus been a major issue for top coat formulation. OEMs have established their own specifications with specific methods and targeted values for the coefficient of friction. Figure 5 shows the results on ten M10 screws coated with Zn/Ni/passivate and a top coat to control the coefficient of friction in the 0.10-0.16 range as specified by GM.
Tapping screw applications require a low coefficient of friction and for the tapping torque to be maintained under critical values. Most present lubricants ensure the proper lubrication, but provide no corrosion resistance. Latest improvements in top coat development have enabled the combination of controlled low coefficient of friction and high corrosion resistance, fulfilling automotive requirements. The frictional properties of one such system are shown in Fig. 6.
Friction on aluminum
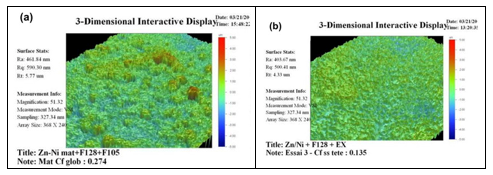
It has been clearly established that the friction of top-coated and trivalent passivated zinc/nickel leads to high friction in contact with aluminum AS5U3 unless certain conditions are met.8 Aluminum, with a hardness close to 90 HV, has rather low friction properties. In contact with the top coated and passivated zinc/nickel, the coefficient of friction on aluminum can suddenly become extreme and lead to damage to the aluminum surface, as shown in Fig. 7.
The solution involves addressing all three deposit layers (i.e., zinc/nickel, passivate and top coat):
- By altering the metallic structure of zinc/nickel by decreasing nodule formation, as shown in Fig. 8.
- By providing a passivate layer capable of retaining the lubricant.
- By acting on the top coat recipe as well. The hydrophobic lubricant must be better linked with the passivate layer and the surface energy of the passivate must be compatible with the top coat.
Finally the best results were achieved with the following structure of deposits and the process was approved by Renault and PSA Peugeot Citroën (see roughness profiles in Fig. 8).
Paint adhesion
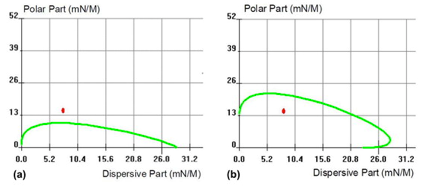
Standardization of parts has resulted in some items having to be treated with a stand-alone treatment or being further coated with paint. The stand-alone treatment should fulfill anti-corrosion requirements and also provide good paint adhesion. A zinc plated part with a Cr(III) conversion treatment cannot fulfill the severe anti-corrosion requirements such as those in the building industry. It has to be protected with a top coat. This top coat contains binder and is hardly receptive to a subsequent paint treatment. Accordingly, work has been directed toward improving the ability of the top coat surface to offer proper adhesion to a paint treatment. Methods such as contact angle measurement enable understanding of the adhesion mechanism and show ways to improve adhesion (Fig. 9).
The wetting envelope of top coat layers were recorded (area under the green line) while polar and dispersive components of subsequent paint were reported on the same graph (red point), as shown in Fig. 10. By adapting the top coat layer composition, it was possible to obtain proper wetting of the top coat layer (Fig. 10b) and favor good paint adhesion on this surface.
Recent developments to face REACH issues
The start of REACH regulation enforcement reinforces the difficulties in placing preparations based on the use of CMR (carcinogenic, mutagenic or reprotoxic) products on the market. Recent EU Commision Directive amendments9,10 state that most cobalt salts are considered to be Category 2 carcinogens. One phrase associated with cobalt dinitrate for concentrations over 0.01% is, “can provoke cancer by inhalation.” Although no directive prohibits the use of these substances, it is strongly recommended to find alternatives to the use of these products.
Cobalt salts are of primary concern in Cr(III) conversion solutions for zinc and zinc alloys. The role of cobalt in Cr(III) conversion solutions has been discussed in the literature. It is used as a metallic inhibitor like most of the transition metals (Ni+2, Fe+2…) and also as a catalyst to enhance the conversion mechanism. GDOES profiles of a cobalt-containing Cr(III) conversion solution shows that cobalt is incorporated in the conversion layer following the same profile as chromium.
The use of silica combined with Cr(III) in the conversion solutions reinforces its anticorrosion properties. However, metallic inhibitors have to be included in the conversion electrolyte to obtain corrosion performance equivalent to that of cobalt-containing passivates. One can see from the profile in Fig. 11 that silica is still incorporated in the layer and another element referenced as “‘A’ element” (light green line) is codeposited along with chromium.
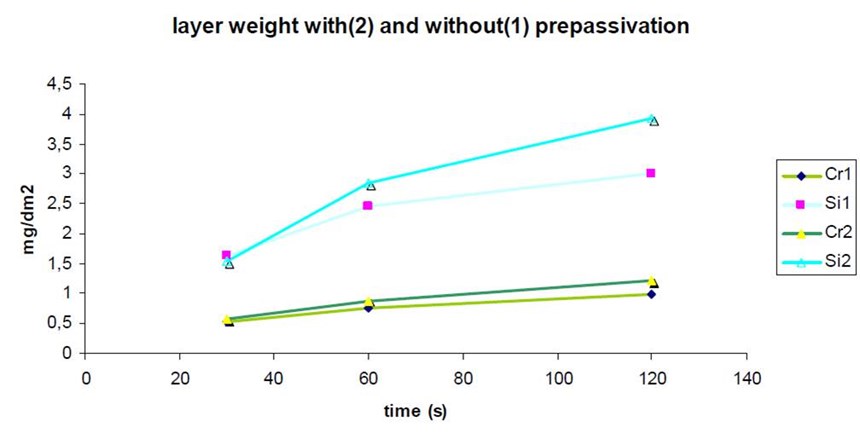
Since the first use of non-Cr(VI) containing passivates in the surface finishing industry, it has always been an issue to ensure proper activation of the substrate. Zinc oxidizes rapidly in air, creating sites where the conversion layer will not form homogeneously. This is in general revealed by formation of black spots during corrosion testing and to a certain extent, premature white corrosion appearance in salt spray test. It is critically important to minimize the exposure time of the zinc layer to air and also to optimize the effectiveness of the rinsing procedure. These measures are not necessarily sufficient and it is often necessary to introduce a “pre-passivation” step. The pre-passivation solution contains trivalent chromium, and forms a thin conversion layer to produce better adhesion for the subsequent conversion layer (Fig. 12).
Conclusion
Clear passivates and soon black passivates on pure zinc are available in the market. They represent a step forward in the direction of the use of non-hazardous substances in the electroplating industry. This same trend is foreseen on zinc alloys and is already available on Zn/Ni. The next challenge may well be nickel itself, as this element is also in the scope of new regulations for the limitation of CMR substances.
References
1. “Directive 2000/53/EC of the European Parliament and of the Council of 18 September 2000 on End-of Life Vehicles,” Official Journal of the European Communities, L269 (21/10/2000); p. 34.
2. “Directive 2002/95/EC of the European Parliament and of the Council of 27 January 2003 on the Restriction of the Use of Certain Hazardous Substances in Electrical and Electronic Equipment,” Official Journal of the European Union, L 37/19 (13/02/2003).
3. “Directive 2002/96/EC of the European Parliament and of the Council of 27 January 2003 on Waste Electrical and Electronic Equipment (WEEE),” Official Journal of the European Union, L37/24 (13/02/2003).
4. “Regulation EC N° 1907/2006 of the European Parliament and of the Council of 18 December 2006 Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH),” Official Journal of the European Union, L396 (30/12/2006).
5. C. Barnes, et al., Trans. Inst. Met. Finish., 60, 45 (1982).
6. M-P. Gigandet & L. Thiery, Techniques de l’Ingénieur, Ref. M1559 (10 December, 2004).
7. M-P. Gigandet, Thesis: “Conversion chromique noire sur dépôts électrolytiques de zinc pur et zinc alliés. Caractérisation, formation et comportement de la couche par analyse statistique” (“Black Chromate Conversion on Electrodeposited Pure Zinc and Zinc Alloys: Characterization, Formation and Behavior of the Layer by Statistical Analysis”), Université de Franche-Comté, Besançon, France (1995).
8. J.J. Duprat & M. Kelly, Fasteners Technology International, 32 (4), 56 (2009).
9. “Commission Directive 2008/58/EC of 21st August 2008 Amending for the Purpose of its Adaptation, for the 30th Time, Council Directive 67/548/EEC,” Official Journal of the European Union, L246/1 (15/9/2008).
10. “Commission Directive 2009/2/EC of 15 January 2009 Amending, for the Purpose of its Adaptation to Technical Progress, for the 31st Time, Council Directive 67/548/EEC,” Official Journal of the European Union, L11/6 (16/1/2009).
RELATED CONTENT
-
Sizing Heating and Cooling Coils
Why is it important for you to know this?
-
Plating Q&A: Can you color stainless steel?
Our expert, Art Kushner, says yes, you can color stainless steel, but it is not a process that is typically performed in a plating shop. Read more about his answer.
-
Copper Plating on Aluminum and Aluminum Alloys
How can I plate copper on aluminum?