Over the years, research on trivalent chromium passivation (TCP) has expanded into many fields of application, among them as a replacement for conversion coatings containing hexavalent chromium (Cr6), known as chromates. REACH-compliant TCP processes have been found to be adequate replacements for chromates, however, implementing them in the highly safety-minded aviation industry, for example, requires fundamental knowledge about the layer composition of these types of conversion coatings.
This article outlines the formation of layers in trivalent chromium (Cr3) passivation over the copper-containing aluminum alloy EN-AW 2024. To derive the reaction processes that take place at the surface as a function of time, we analyzed the topography and the chemical composition of the coating, and electrochemically measured the corrosion potential during the conversion process.
Featured Content
The topography of the surface was determined by scanning electron microscopy (SEM) combined with energy-dispersive X-ray (EDX) spectroscopy. X-ray photoelectron spectroscopy (XPS) was used to analyze the chemical composition of the layer. XPS measures only the outermost surface, and besides defining the elements present, can be used to derive their oxidation states and their chemical structures.
To follow the layer formation as a function of time, surfaces were analyzed after different immersion times in the passivation solution. The results were compared with the free corrosion potential measured in situ during the conversion process with a Luggin capillary. This allowed us to derive a model of the layer formation.
SEM/EDX Analysis: Topography
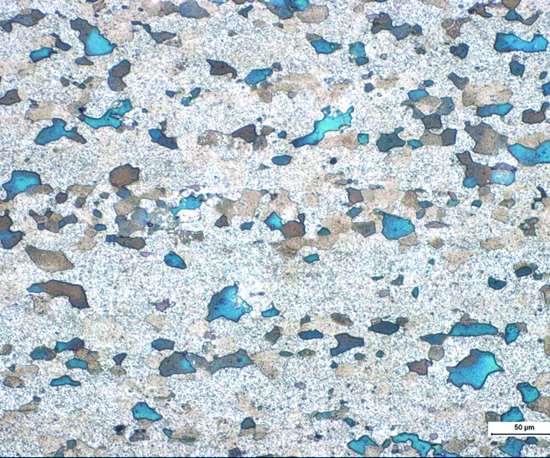
Figure 1 shows the 2024 alloy before passivation. The surface was processed in a sequence of chemical treatments typical for preparing a surface for passivation: mild alkaline degreasing, alkaline etching and acid desmutting. Notice the etching pits and exposed copper phases (dark areas) detected by EDX measurement. The etching pits have a diameter of 20 to 50 microns, whereas the copper phases are 6 to 30 microns in size.
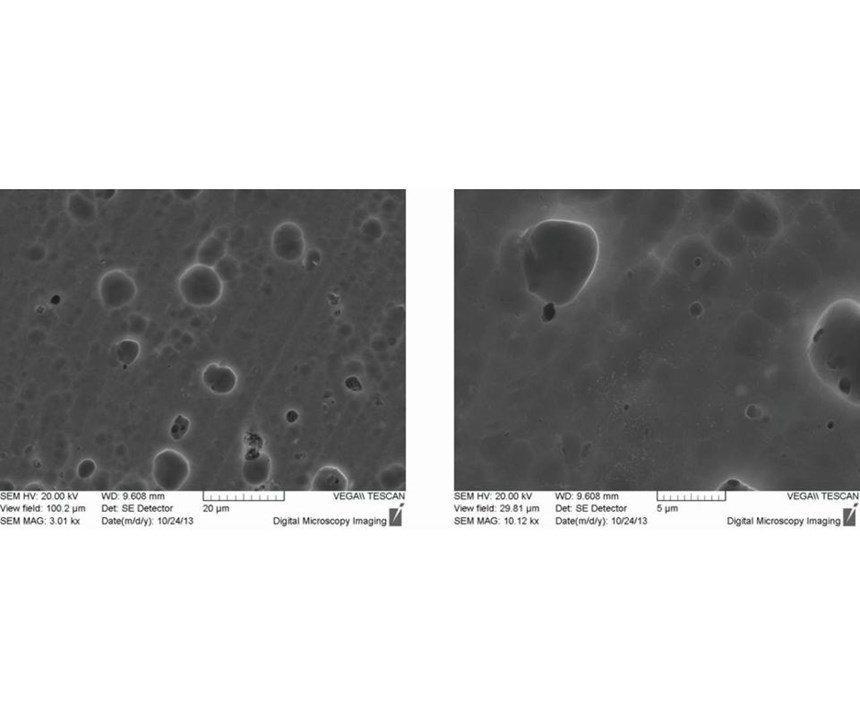
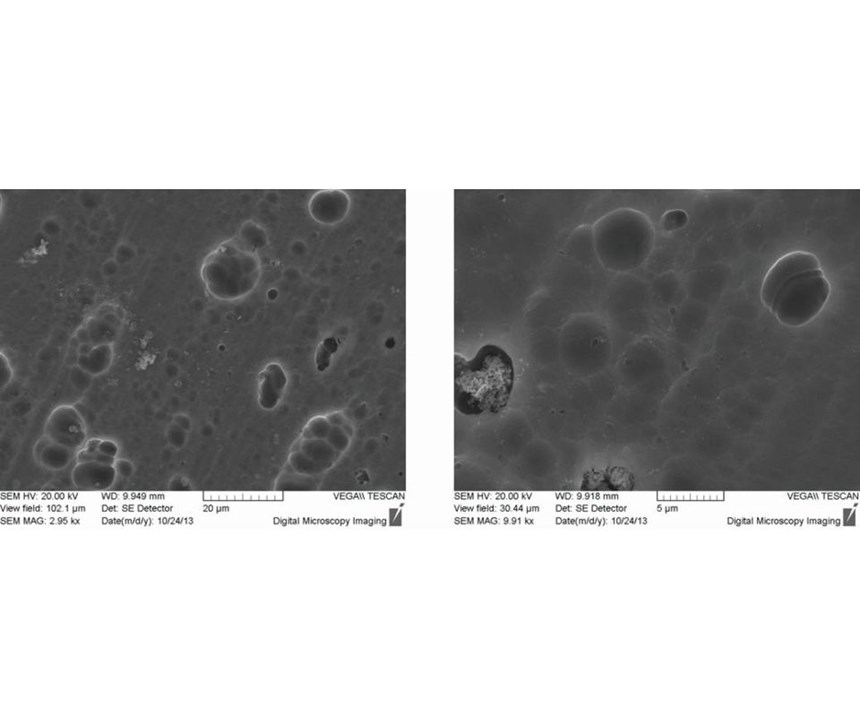
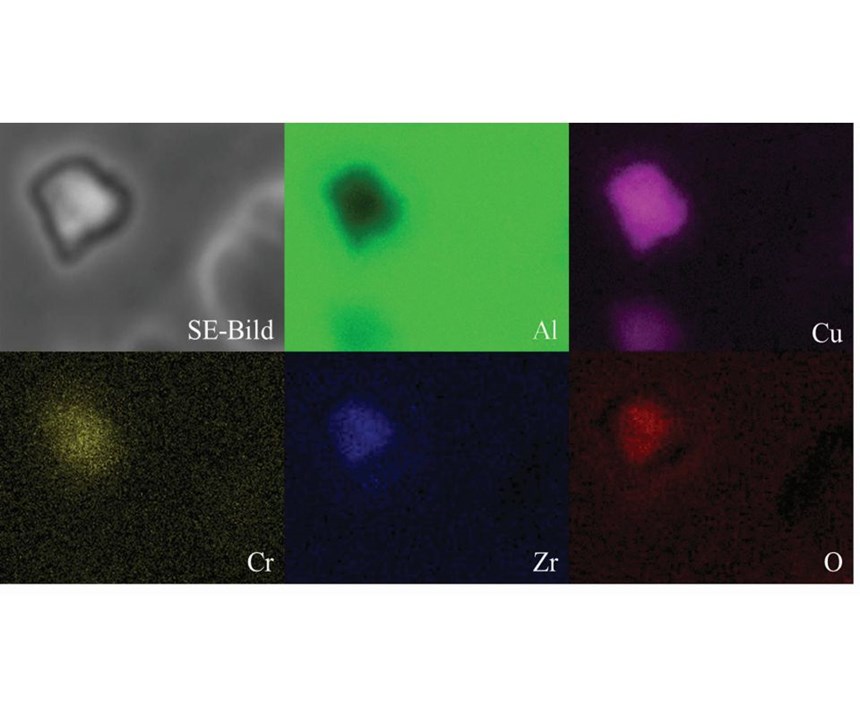
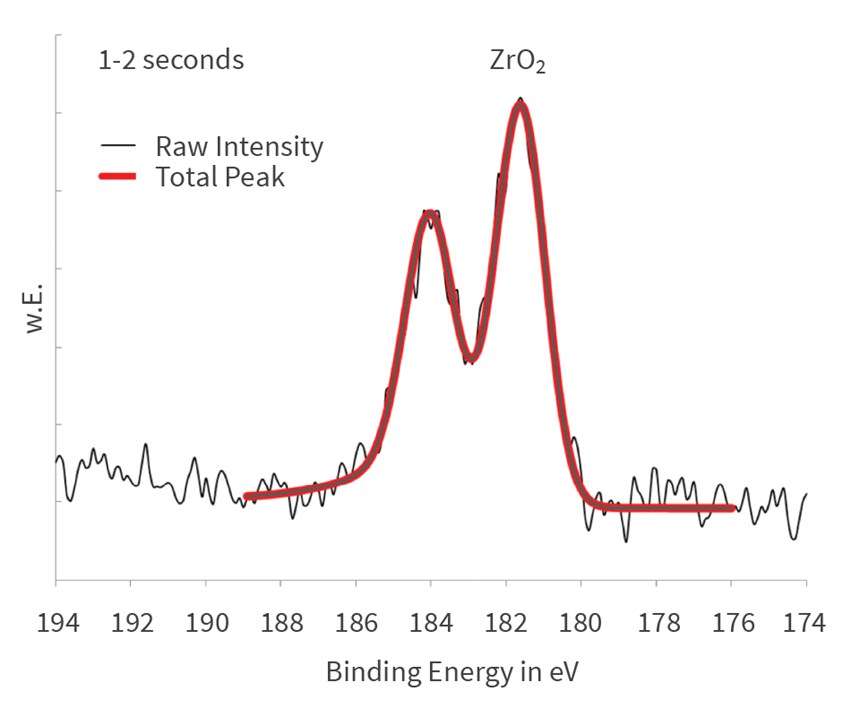
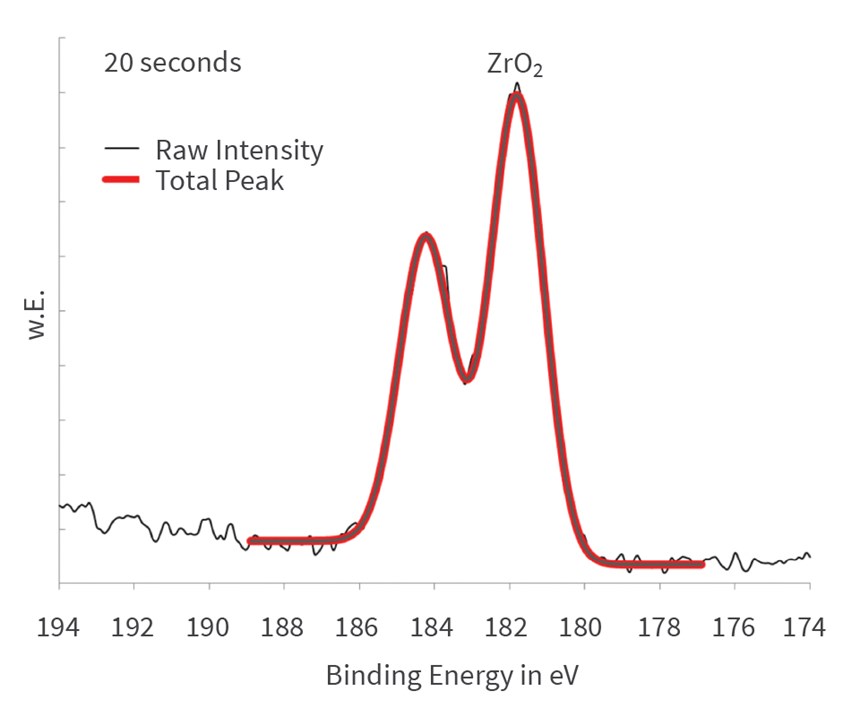
After 1-2 seconds immersion in the passivation bath, the copper phases can no longer be detected. Instead, light gray structures are visible (Figure 2). EDX detected zirconium, chromium and oxygen at these sites. In addition to this spot analysis, the area around a copper precipitate was analyzed by EDX mapping (Figure 3). The colored maps of aluminum and copper testify that the passivation layer initially grows on the copper precipitate. Chromium, zirconium and oxygen predominantly are deposited in this area. After 20 seconds immersion, the growing passivation film can be detected in other areas as well. Note the bright particles with a diameter of approximately 0.1 micron. EDX spectra of these particles merely show zirconium and oxygen. Additionally, after 20 seconds, microcracks started to form in the precipitated passivation layer in some areas (Figure 4).
During further processing, more particular structures formed, and locally defined microcracks occurred. Beginning at 180 seconds, laminar cracks started to form. Figure 5 shows the surface after 300 seconds immersion time.
XPS Analysis: Composition
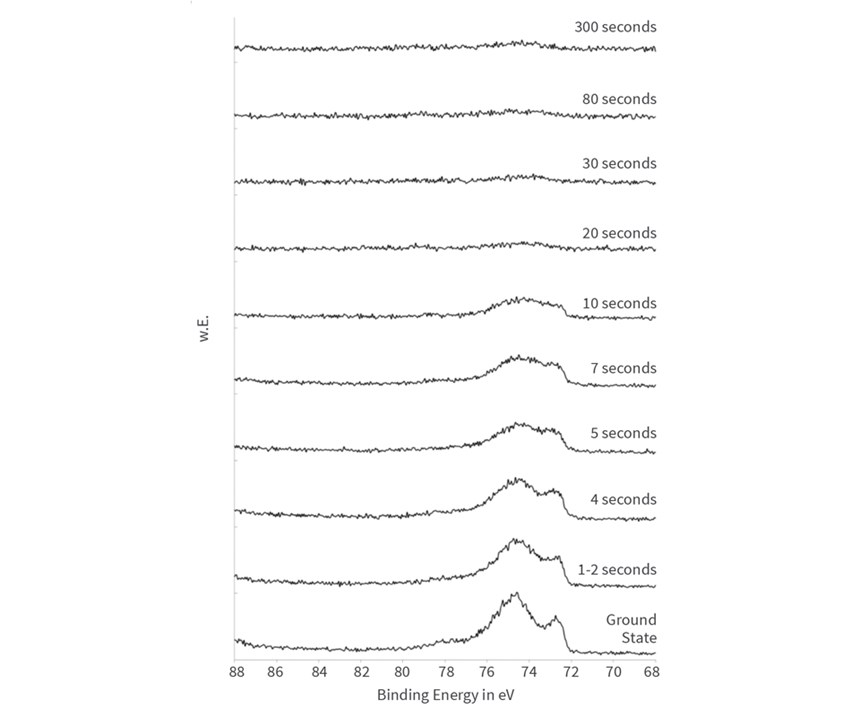
During passivation, zirconium acts as a layer-forming adsorbate element, and therefore the intensity of the emission rises with the immersion time (Figure 6). The detailed spectra (Figure 7) show that zirconium is present as Zr4+-oxide throughout the whole passivation process.
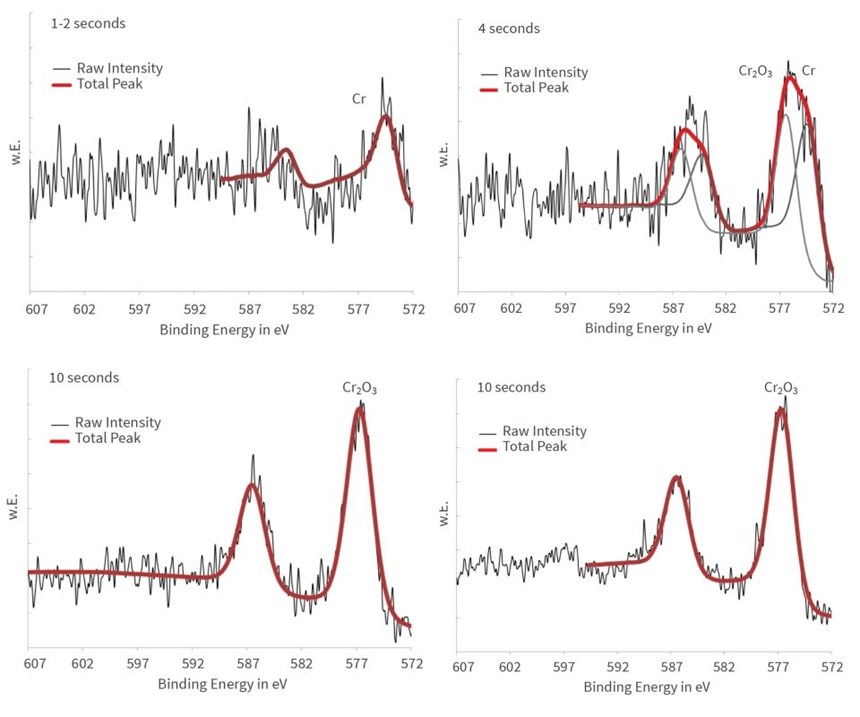
The spectra of the chromium adsorbate element are especially informative. As in the case of zirconium, the intensity of its emission increases with the longer immersion time. Notable is the binding energy shifting from 574 electron-volt (eV) at very short immersion times to 577 eV at longer immersion times, which means the chromium is deposited in different compositions and at different oxidation states during the reaction time.
The detailed spectra are shown in Figure 8, revealing that the chromium cements in metallic form on the aluminum substrate during the first few seconds. After 10 seconds, the metallic state is not traceable anymore in the spectra. Obviously, the metallic chromium is covered by chrome(III) oxide (Cr2O3) at longer immersion times.
The question of whether Cr6 compounds might evolve during trivalent chromium passivation has been raised, however, wet chemical analyses have shown no traceable Cr6, and XPS analysis indicates only trivalent chromium compounds next to metallic chromium.1 In fact, the binding energy of Cr6 compounds would be significantly higher at approximately 579 eV. Distinctively, the process deposits only trivalent chromium(III) compounds.
Free Corrosion Potential
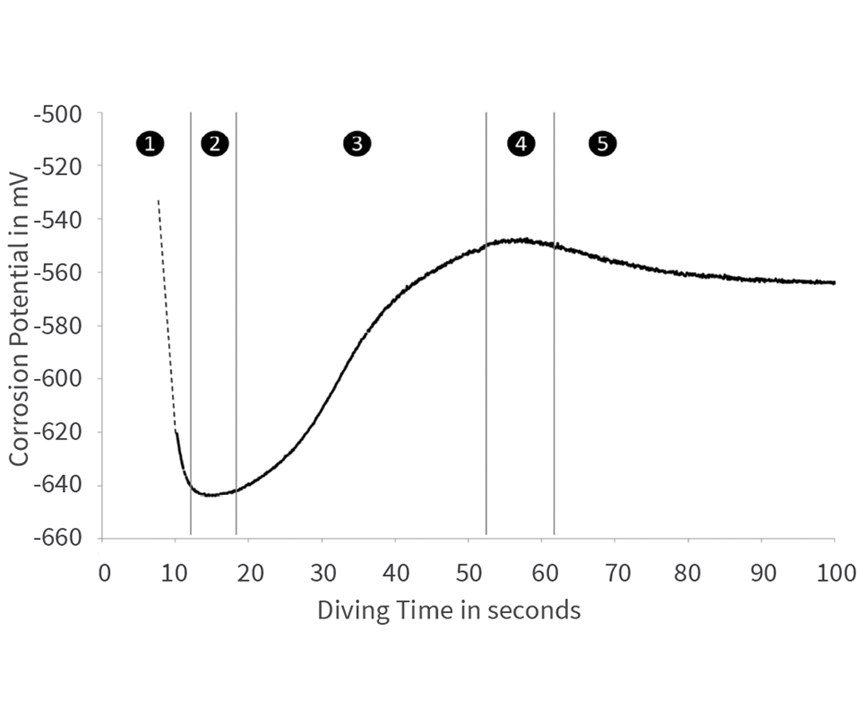
Figure 9 shows measurement of free corrosion protection during passivating of EN-AW 2024. The measurement curve can be divided into five phases. Until 10 seconds immersion, the potential decreases significantly. This indicates a fast conversion of substances at the surface. If this effect is correlated to the results from SEM/EDX and XPS, we can conclude a strong pickling reaction and simultaneous fast deposition of a first passivation layer.
During the second phase, 10-20 seconds immersion, the conversion layer densely covers the surface. In particular, the highly reactive copper phases are completely covered with Zr4 and Cr3 oxides, and the potential curve is at a minimum, without significant conversion of matter.
After about 20 seconds (phase three), small, thin cracks form in the passivation layer. They enable an exchange of substances and further conversion processes. The corrosion potential increases, and the passivation layer becomes thicker.
The maximum height of the curve in phase four cannot be sufficiently explained. Since it is still a phase of minor, but relatively constant conversion processes, it can be assumed that the microcracks are too small for a faster-proceeding reaction.
Phase five starts after about 70 seconds. Gradually, bigger cracks form in the layer, again allowing a higher conversion of matter to proceed at a steady pace.
Correlation of the Results
Comparing the free corrosion potential and the XPS measurements is particularly interesting for the elements aluminum and chromium.
Aluminum was detected in three different states by XPS: as metallic, as fluoride and as oxide. The intensity of the metallic aluminum increases until about 5 seconds reaction time, because the bulk of the material is revealed by the pickling reaction. After about 10-20 seconds, no aluminum metal is detectable at the surface, indicating that the layer is densely formed, covering the entire surface. The near-surface concentration of aluminum oxide and fluoride decreases until 20 seconds reaction time and remains constant afterwards. The aluminum is transported to the outer surface through the microcracks and settles as aluminum oxide and fluoride, incorporated in the passivation layer.
The chromium is deposited as metallic during the first few seconds of immersion. It can be concluded that the cementation of the chromium, which is electrochemically more noble than the aluminum, takes place on the metallic aluminum surface. Simultaneously with the signal of the aluminum metal, the signal of the metallic chromium vanishes after 10 seconds. The concentration of chromium(III) oxide increases steadily until about 20 seconds and remains constant afterwards in the growing passivation layer. Cr6 compounds are not detected at any time.
Conclusion
By chronologically monitoring the passivation reaction—measuring the free corrosion potential by XPS and SEM combined with energy disperse EDX—we were able to define the chemical reactions that occur during the passivation process in more detail and generate a scheme for the layer formation.
Initially, before the passivation process, the aluminum surface was primarily metallic, with parts oxides and fluorides caused by the desmutting process. Copper in inter-metallic phases was detected as metal as well as oxide. During the first 10 seconds of passivation, a strong and fast conversion of material took place. Pickling reaction and layer formation were in strong competition. Metallic aluminum and aluminum oxide were attacked and dissolved. Chromium was deposited as a metallic in a cementation reaction:
Al2O3 + 6H3O+ → 2Al3+ + 9H2O
2Al + 6H3O+ → 2Al3+ + 6H2O + 3H2
2Cr3+ + 3H2 + 6H2O → 2Cr + 6H3O+
(reactions during the first 10 seconds)
The copper-rich phases of the 2024 alloy are especially reactive. SEM/EDX detected the strongest structural change, and the phases were covered quickly with deposit.
After 10 seconds, the surface was covered completely by the conversion layer. Metallic aluminum and copper of the bulk material were not detectable by XPS. After about 10-20 seconds, small microcracks formed in the passivation layer, predominantly in the vicinity of the copper phases, enabling an exchange of material and leading to further layer growth. Mixed oxides were deposited, and aluminum was dissolved by acid and fluoride attack and was incorporated into the layer. After about 50 seconds, pure Zr4 oxide was detectable,
especially next to the grain boundaries of the copper phases.
2Al + 6H3O+ → 2Al3+ + 6 H2O + 3H2
xCr3+ + yZr4+ + zAl3+ + nOH- → CrxZryAlz(OH)n
Zr4+ + 4OH- → ZrO(OH)2 + H2O
(significant reactions after 20 seconds)
At longer immersion times, bigger cracks formed, allowing more material exchange and further growth of the passivation layer.
The final layer essentially consisted of chromium(III), zirconium(IV), and mixed aluminum oxides. These oxides are extremely resistant and inert, and provide a good barrier
against corrosive atmospheres.
Especially, the formation of metallic chromium during the first seconds of the reaction needs to be pointed out. The passivation at the aluminum surface is deoxidizing, and hexavalent chromate is not formed during the process. Trivalent chromium(III) compounds fulfill the demands of REACH, WEEE2 and ELV3 directives.
1S.L. Suib, J. La Scala, W. Nickerson, A. Fowler, N. Zaki, “Determination of Hexavalent Chromium in NAVAIR Trivalent Chromium Process (TCP) Coatings and Process Solutions,” Metalfinishing, Feb. 2009
2Directive 2002/95/Ec of The European Parliament and of the Council, Jan. 2003
3Directive 2000/53/EC of the European Parliament and of the Council, Sept. 2000
Jennifer Honselmann and Eric Mankel are with the University of Darmstadt; Peter Volk is with SurTec. Visit surtec.com.
RELATED CONTENT
-
Stripping of Plated Finishes
The processes, chemicals and equipment, plus control and troubleshooting.
-
Aluminum Anodizing
Types of anodizing, processes, equipment selection and tank construction.
-
Nickel Electroplating
Applications, plating solutions, brighteners, good operating practices and troubleshooting.