Anodization of aluminum provides mechanical and chemical protection to aluminum metal and alloys by building a porous aluminum oxide layer, which is then sealed by chemical and/or physical methods. Structural parameters (including overall coating thickness, pore size (radius), pore wall thickness and pore length) are dependent on a number of factors, including anodize time, current density, tank temperature and electrolyte concentration. For example, increased current density results in larger pores and thicker walls, whereas higher temperatures lead to lower coating thickness due to an increased dissolution rate of the anodize layer. These conditions can also affect compositional parameters (for example, higher anodize temperatures lead to more incorporation of sulfate impurity anions in the layer). For the safest conditions, we generally recommend a tank temperature of 70°F and anodization at a current density of 18 A/ft2 (ASF).
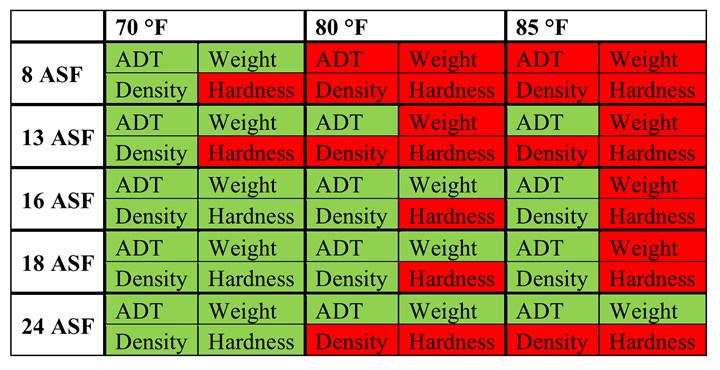
When an anodized part fails one of the tests used to evaluate anodize quality, sealing is often pointed to as the culprit. However, due to the factors mentioned above, the quality of the aluminum oxide layer can depend heavily on conditions in other tanks such as the anodize tank. To probe the effects of some anodize conditions and to learn about their practical limits with respect to passing performance tests, we looked at Class I anodized parts using standard anodize parameters, only varying anodize tank temperature and anodizing current density, and looked at its effects on acid dissolution test (ADT) results, coating weight, coating density, and Michael-Clarke abrasion resistance.
Featured Content
Acid Dissolution Test
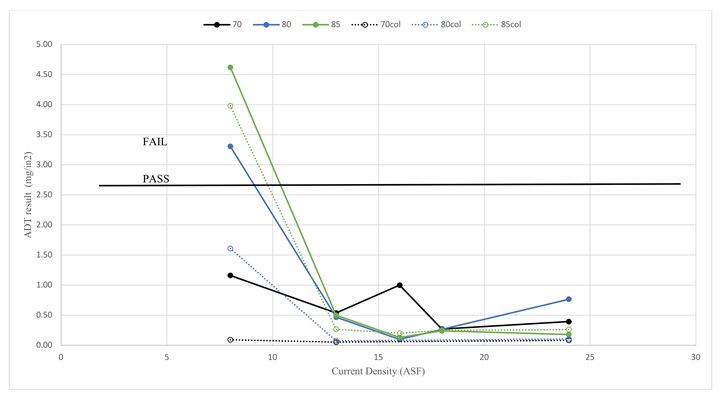
The ADT is the only test in the AAMA 611 specification explicitly designed to test seal quality, and the results of this study bear that out. Figure 1 shows the results of the acid dissolution test (ADT) against the current density at anodize tank temperatures of 70°F, 80°F and 85°F. Immediately apparent is that at 13 ASF and above, all parts pass the 2.6 mg/in2 requirement, regardless of the bath temperature. At 8 ASF, ADT results show a clear trend with high bath temperatures corresponding to failure of the ADT, which indicates that the ADT fails to indicate seal quality only at the lowest current densities and that higher temperatures during anodizing lead to a poorer quality coating. Also, at 8 ASF, electrolytically colored parts performed better than their clearcoated counterparts. Typically, industrial processes find the opposite trend, but any number of experimental variations may account for this reversal and, in this case, is likely due to invisible surface smut from very short rinse times and unfiltered seal bath. Further trends are difficult to discern due to the large variance in ADT results. Unexpectedly, the 80°F/8 ASF/color parts pass, but, for this set of conditions and also for 85°F/8 ASF/clear, the variance is extremely high with values (5.63, 0.98) and (2.82, 0.39), respectively.
Coating Weight And Density
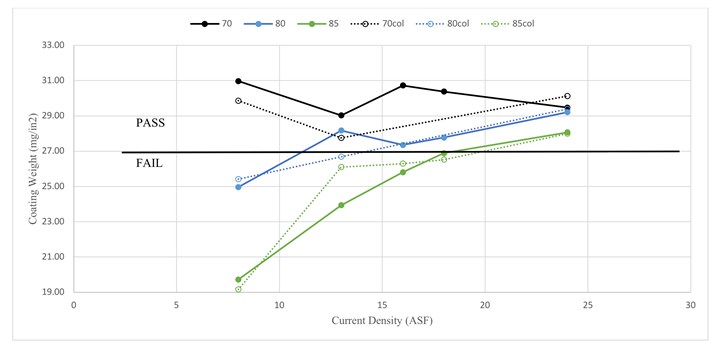
For coating weight, bath temperature appears to have a greater impact than current density with electrodeposition of tin having no discernable trend in its impact (Figure 2). Generally, higher anodize temperatures and lower current densities result in poorer coating weights and only at 70°F or at 24 ASF are reliably passing results obtained with room for error. Recall that the aluminum oxidation and oxide dissolution rates are primarily responsible for the coating weight, and the reaction and dissolution of the building anodic aluminum oxide with the concentrated acid in the anodize tank is responsible for the lower coating weights at higher temperatures.
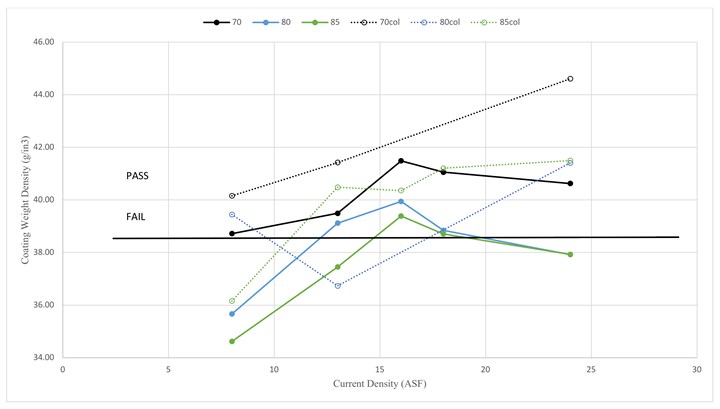
Reliable passing values for coating weight densities are only obtained when anodizing at 70°F (Figure 3). Recall that at higher temperature, the sulfuric acid electrolyte reacts more quickly with the aluminum oxide that is being built during anodization. The exact phenomena behind these results are not explored here, but could be a result of dissolution inside of the pores and/or uneven dissolution at the surface, which would lead to minimal impact on the overall layer thickness while still reducing the coating weight and, therefore, density.
At all temperatures, coating density rises with current density up to 18 ASF, before gradually decreasing. At the highest current densities, the pore structure of the anodized aluminum oxide trends toward thicker cell walls and larger pores. This trend, combined with the shorter anodize times, results in lower layer density at high current densities. At the lowest current densities, other factors (such as incorporation of electrolyte and lower rate of oxidation) outcompete the longer anodize times, resulting in lower layer density. As a result, the optimal current density for coating weight density is 16 ASF, with 18 ASF also safe.
Generally, coating densities for colored parts are higher than their clear counterparts and can be attributed to electrodeposition of tin contributing to the mass without otherwise affecting the anodize layer under these conditions.
Michael-Clarke Abrasion Test
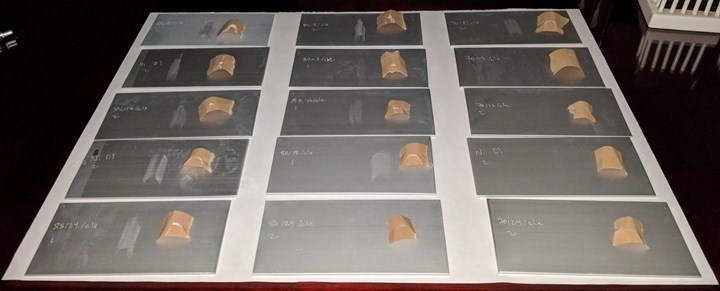
Michael-Clarke abrasion tests fail on all parts anodized at 85 or 80°F; very noticeably for parts at 85°F or at low current density; and at 70°F for parts anodized at 8 ASF (Figure 4). As expected, higher temperatures and lower current densities lead to poor mechanical properties, with the part anodized at 85°F/8 ASF showing an obviously powder-soft coat. These results are consistent with the general trends found for coating weight density and can be attributed to the same underlying microstructural issues of thinner pore walls and more chemical inclusion into the aluminum oxide pore walls. The physical reasoning for thin walls with small pores failing and thick walls with large pores passing can also be applied at the macroscale. For example, the Parthenon would be expected to be much less stable if each of the columns were replaced by many more slender beams.
Take-Home Messages
- The only AAMA 611 test that reliably indicates a problem with sealing is the ADT.
- This test gives a false negative at very low current densities.
- Coating weight only reliably passes at 70 °F. Coating weight density passes reliably at 70°F, but values go down at very high and low current densities.
- 18 ASF and 70°F offer a very safe region for good results in all of the performed test from AAMA 611.
- The sensitivity and simplicity of the Michael-Clarke abrasion test make it an excellent first indicator of poor conditions in the anodize tank.
- However, the abrasion test failure CAN be a result of poor sealing and the results of this test (and other tests) must be considered in conjunction with other tests to determine the root cause of quality test failures.
George N. Oh, Ph.D., and Nathan Sheffield are chemists with Houghton International’s metal finishing division.
REFERENCES
Runge, Jude Mary (2018). The Metallurgy of Anodizing Aluminum: Connecting Science to Practice, pp160-187. Springer International Publishing AG, Cham, Switzerland.
Brace, Arthur W. and Sheasby, Peter G. (1979). The Technology of Anodizing Aluminum, 2nd ed. Technicopy Ltd, Gloucestershire, England.
American Architectural Manufacturers Association (2014). AAMA 611-14: Voluntary Specification for Anodized Architectural Aluminum. Schaumburg, IL.
RELATED CONTENT
-
How to Apply the 720 Rule to Current Density Anodizing
What can you tell me about the 720 Rule as it applies to current density anodizing? Plating expert Sjon Westre, Ph.D., from Chemeon, answers this question.
-
Aluminum Anodizing
Types of anodizing, processes, equipment selection and tank construction.
-
Aluminum Surface Finishing Corrosion Causes and Troubleshooting
In this paper, a review of several process solutions, examining coolants, solvent cleaning, alkaline clean/etch and deoxidizing/desmutting, listing intended and unintended chemical reactions along with possible mechanisms that would favor corrosion formation.