Some Engineering Aspects of Electrodeposition - The 6th William Blum Lecture
This paper is a re-publication of the 6th William Blum Lecture, presented at the 51st AES Annual Convention in St. Louis, Missouri, on June 15, 1964.
#basics
by
Mr. R.A.F. Hammond
Featured Content
Recipient of the 1963 William Blum
AES Scientific Achievement Award
Originally published as
Annual Technical Proceedings of the American Electroplater’s Society, 51, 9-20 (1964).
Editor’s Note: This paper is a re-publication of the 6th William Blum Lecture, presented at the 51st AES Annual Convention in St. Louis, Missouri, on June 15, 1964. A printable PDF version is available by clicking HERE.
ABSTRACT
The successful use of electrodeposits in engineering applications, including corrosion protection, depends not only on the properties of the coatings themselves but upon their influence on the mechanical properties of the basis metal. In this connection, three possible effects must be considered: (1) on the static tensile properties, (2) on the fatigue strength and (3) on the sustained-loading properties (hydrogen embrittlement). Unless suitable precautions are taken serious loss of fatigue and sustained-loading properties may result, particularly of high-strength steels, and the increasing use of such steels in the aircraft industry has highlighted this problem. As a result, much research effort has been concentrated on these subjects in recent years and the lecture reviews some salient features of current knowledge in this field.
Although electrodeposition has been practiced industrially for over a century, it is only in the last 50 years or so that the subject has been studied scientifically. In this development Dr. William Blum, in whose honor this series of lectures was inaugurated, was an outstanding pioneer.
As so frequently happens, war provided an early stimulus for electrodeposition research and, on consulting the very long list of Dr. Blum's published work, I found that, although his first two electrodeposition papers were on its application to the peaceful art of printing, his third was entitled "Military Applications of Electroplating" (1918).
World War I also provided the stimulus for the formation of the Electrodeposition Section of the Research Department, Woolwich (now Royal Armament Research and Development Establishment, Ministry of Defence, Army Department) in which I have been privileged to serve for most of my career. This arose directly from a British Army development about the year 1916 in which a plating shop for the reclamation of gun parts and other Services components was set-up behind the lines at the Le Havre Base-Workshops of the Royal Ordnance Corps. A parallel war-time development was initiated by the Royal Flying Corps (predecessor of the Royal Air Force).
These activities were, I believe, the very first examples of the application of thick electrodeposits for engineering purposes - for the first time the engineer had acquired a "putting-on tool."
However, success in this new field depended upon securing very high adhesion, freedom from defects and controlled mechanical properties of thick deposits (then usually copper, iron or nickel) - matters which up to that time had not received very much attention. Early work at Woolwich was therefore concentrated on these aspects. Much early work on the structure and properties of electrodeposits was also done by Dr. Blum at the National Bureau of Standards, Washington, D.C. and, somewhat later, the classic researches of Dr. Abner Brenner and his colleagues on chromium and nickel appeared, sponsored I believe by your Society under its Research Scheme. This work is of special importance in relation to the subject of my lecture.
These and other investigations have provided a great body of information on the properties of the deposits themselves. However, it is only more recently that attention has been concentrated on the influence of the deposits on the mechanical properties of the substrate material and this has arisen largely owing to the increasing use of electrodeposition for salvaging, hard-surfacing or corrosion protection by the aircraft industry where any failure could have serious consequences. Such failures are all too possible unless finishing techniques are used with discrimination, based upon sound knowledge of the methods involved. In the large majority of such applications, steel is the substrate material and, as the potentially harmful effects of pickling and plating processes tend to increase with the tensile strength of the steel, the importance of the subject has become even greater in recent years with the increased utilization of high and ultrahigh strength steels. For this reason, during the last 15 years or so, there has been a great concentration of effort to determine the effect of plating processes on the mechanical properties of the substrate.
It has been my aim in preparing this lecture to provide, in a compact form, an account of the salient features of current knowledge in this field, particularly in regard to coatings of chromium and nickel which are the deposits normally used for engineering applications, and to cadmium now so extensively used for corrosion protection.
In considering the possible effects of such processes, three aspects must be taken into account: (1) the effect on the static tensile properties, (2) the effect on fatigue strength and (3) the effect on sustained-loading properties due to hydrogen embrittlement. These will be discussed in turn.
STATIC TENSILE PROPERTIES
Chromium
Electrodeposited chromium is a hard, brittle material which normally contains internal cracks. It cannot therefore be relied on to contribute strength to the component proportionately to the steel which it replaces. The coatings used are normally quite thin, however, and the quota of strength required of the chromium is insignificant. Nevertheless the possibility must be considered that the presence of the chromium may adversely affect the properties of the steel itself. This possibility was investigated by Logan1 who determined the effect of chromium plating on the mechanical properties of SAE 4130 steel of 187,000 psi. His trials included tensile, tensile impact, bending and crushing tests and he found that, although the tensile and yield strengths of plated specimens decreased as the plate thickness was increased from 1 to 15 mil, these properties did not fall below 91 per cent of the values of the unplated steel. The plastic deformation before fracture in the tensile tests became less as the plate thickness increased but baking at 200 or 440°C (392 or 824°F) for various times restored the ductility to a value approaching that of the unplated steel and it seems likely that hydrogen embrittlement of the steel was the cause of the reduced ductility.
It seems reasonable to conclude, therefore, that the presence of a chromium coating per se will not impair the tensile properties of a steel under any normal conditions of service.
Nickel
The electrodeposition of nickel for repair or salvaging purposes is used much more extensively in the U.K. than in the U.S.A. In contrast to hard chromium plating, the deposits are frequently of very substantial thickness and, in exceptional cases, the cross-sectional area of the nickel coating may represent a sensible proportion of the whole. The effect of the nickel on the overall strength of the component could therefore be important, particularly in components with a low strength factor. Unlike chromium which usually contains cracks, nickel is a relatively strong material with a high degree of ductility so that a properly applied coating contributes very materially to the overall strength.
Tests to determine this effect quantitatively were made by Hothersall2 who used standard tensile test-pieces of steels of two strengths (Hardness 183 and 286 DPH respectively). These were machined undersize, plated with a surplus of nickel and machined back to size in such a manner that 20 per cent of the finished cross-section consisted of nickel. Two types of nickel (hard and soft) were included in the tests. The specimens were then tensile tested to fracture and the results are given in Table 1.
No significant effect on the ultimate tensile strength or on the ductility of the composite resulted from nickel plating, with the exception that the elongation was somewhat reduced by the harder nickel on the stronger steel.
Table 1 - Effect, upon tensile properties of steel, of replacing 20 per cent of the cross-sectional area with electrodeposited nickel (average results).2
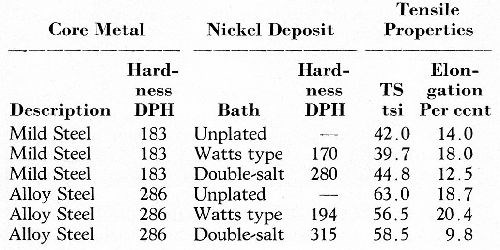
There are good grounds for concluding, therefore, that no serious effect on the static tensile properties of steel will result either from hard-chromium or heavy nickel plating.
FATIGUE STRENGTH
When subjected to fluctuating stresses metals fail by "fatigue," at stress values very much lower than the tensile strength of the metal. In the case of steels, it is possible to define a critical stress (the "fatigue limit") below which the metal will not fail, however, many cycles of fluctuating stress are applied and this value of stress for plain (un-notched) specimens is approximately one-half the tensile strength of the steel.
It has long been known that the application of even a thin electrodeposited coating may drastically reduce the fatigue limit of the substrate. Thus over 30 years ago Barklie and Davies3 published quantitative data on the loss of fatigue strength of steel by nickel plating, showed that it was greater for thick than for thin deposits and postulated that it was associated with internal stress in the deposits (demonstrating the superior properties of low stress deposits). They also advanced a theory as to the mechanism of fatigue failure of coated metals upon which the current interpretations are still based. Briefly, the basic mechanism is as follows: Fatigue is a tensile stress phenomenon and when a tensile stress is applied to a plated component, if the inherent fatigue limit of the coating metal is less than that of the steel substrate, fatigue cracks will form in the coating at a lower applied stress than the fatigue limit of the substrate. The crack in the coating will then act as a notch, concentrating the tensile stress on the surface of the substrate. When the local stress exceeds the fatigue limit of the substrate, the crack will propagate inwards and fatigue fracture will follow. (This mechanism was illustrated diagrammatically in a paper to this Society by my colleague Cyril Williams and myself in 1959.4) It follows therefore that the important factors are (1) the inherent fatigue strength of the coating metal relative to that of the substrate and (2) the magnitude and sign of any internal stress in the coating.
The results of more recent work* on the effects of chromium and of nickel plating are discussed in the light of this theory, consideration being given first to the coating fatigue strength.
Tensile strength and inherent fatigue strength
Chromium
The direct determination of the fatigue strength of electroformed chromium would be extremely difficult owing to the presence of internal cracks. Brenner attempted to determine the tensile strength of chromium deposits but obtained very variable results for the same reason.5 However, an investigation by Williams and Hammond,6 in which the change in fatigue strength of a range of steels of widely differing strengths produced by a 6 mil deposit from the conventional hard chromium solution was determined, gave reasonable grounds for deducing that the inherent fatigue limit of the chromium was approximately 22 tsi.**
Nickel
The only direct determinations of the inherent fatigue strength of electrodeposited nickel known to the author are those carried out by Brenner, Zentner and Jennings.7 These investigators determined the fatigue strength of electroformed nickel sheets in unidirectional bending (zero minimum stress). In nickel deposited from solutions which give the softer grades of metal, the structure and properties are heterogeneous across the section, being relatively hard and fine-grained near the starting surface and softer and coarse-grained at the outer surface. For the Watts and chloride solutions, therefore, two values of fatigue limit are quoted (a) with the outer surface and (b) with the starting surface stressed in tension. Some of the results are given in Table 2 and, as would be expected, the (b) values are substantially higher. However, for the Watts deposit, most commonly used for heavy nickel deposition, the mean value of inherent fatigue strength is approximately 15 tsi. The considerably higher value for the organic bright nickel should be noted.
Table 2 - Mechanical properties and fatigue limit of electrodeposited nickel (Brenner, et al.7).
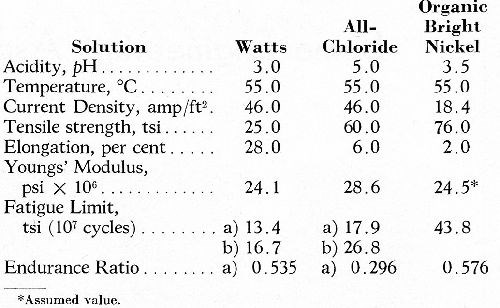
Indirect evidence is also obtainable from the considerable amount of data available for the tensile strength of electrodeposited nickel some of which, extracted from the literature, is listed in Table 3. Reference to Part 2 of the Table ("straight" solutions) provides ample evidence from a number of sources that the tensile strength of nickel deposited from the sulfate, sulfate/chloride, sulfamate or fluoborate baths lies between 25 and 29 tsi. Assuming an average endurance ratio of 0.5*** as found by Brenner (loc cit), the inherent fatigue limit of the nickel from various types of solution is again seen to be approximately 15 tsi.
Table 3 - Comparison of the mechanical properties of nickel deposits produced under broadly comparable operating conditions from various types of plating solution.
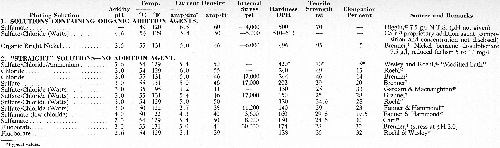
Thus it is seen that the inherent fatigue strengths of chromium and most "straight" nickel deposits are approximately 22 and 15 tsi, respectively. Constructional steels usually lie in the strength range of 60 to 120 tsi (fatigue limit approximately 30 to 60 tsi) and it is evident, therefore, that fatigue failure if it occurs is likely to initiate in the coating. It is also evident that anything that can be done to increase the inherent fatigue limit of the deposits will be valuable from the standpoint of minimizing loss of fatigue strength in plating. In the case of nickel deposits this can be done by the use of addition agents and by other means (see below).
Internal stress
Electrodeposited chromium and nickel usually exhibit internal tensile stress, typical values for the conventional hard chromium and Watts nickel deposits being 4 to 8 and 8 to 10 tsi, respectively.
It is evident that such internal stress will be additive to any applied stress and will therefore tend to diminish the effective fatigue strength of the coating. This in turn will encourage fatigue failure of the plated substrate. In fact it has been found that there is a linear relationship between the loss of fatigue strength and the intensity of tensile stress for both chromium and nickel deposits.
Chromium
In an investigation by Williams and Hammond,6 the linear relationship between internal stress and the change of fatigue strength of chromium plated steel was established in two ways: (1) by determining the change in fatigue limit in the as-plated (unbaked) condition when a steel of 60 tsi tensile strength was plated to a thickness of 6 mil with chromium from a variety of baths giving deposits of widely varying residual stress (the latter being determined independently by the bent-strip method) and (2) by varying the internal stress of standard chromium from the conventional bath by controlled heat-treatment, the stress strips being baked at the same temperatures and for the same times as the corresponding fatigue test-pieces. In this case steels of 60 and 80 tsi tensile strength were tested. The results are shown in Figs. 1 and 2. Similar results were obtained by Stareck, et al.8
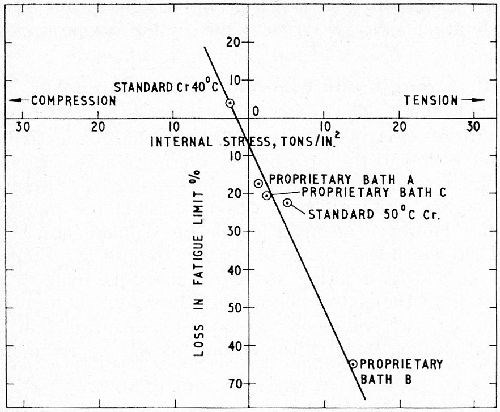
Figure 1 - Effect of the internal stress of chromium from various solutions on the fatigue limit of En 25 steel of D.P.H. 305; 6 mil of chromium “as plated.”6
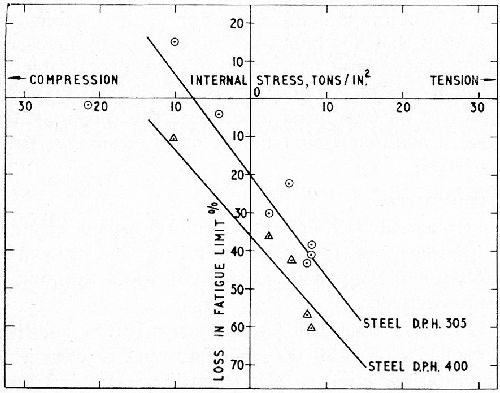
Figure 2 - Effect of residual internal stress in baked chromium deposits on the fatigue limit of En 25 steel of two strengths (Hardness 305 and 400); 6 mil of chromium.6
Nickel
Hothersall and Gardam9 determined the change in fatigue strength of a steel of 43 tsi strength induced by plating 1 mil of nickel from a variety of dull and bright nickel baths giving deposits of varying internal stress. The results, plotted in Fig. 3 (upper curve) clearly show the linear relationship. Also shown on the graph are four additional sets of results from the literature and, although for three of these sources only two points are available and only three points for the fourth, it appears to be significant that the slope of all the curves is substantially the same.
There is thus no question that the internal stress of an electrodeposit is an important factor in determining the extent to which a substrate metal suffers change (usually loss) of fatigue strength on plating. Its importance relative to that of the other main factor (the inherent fatigue strength of the coating) depends very much on the strength of the substrate.
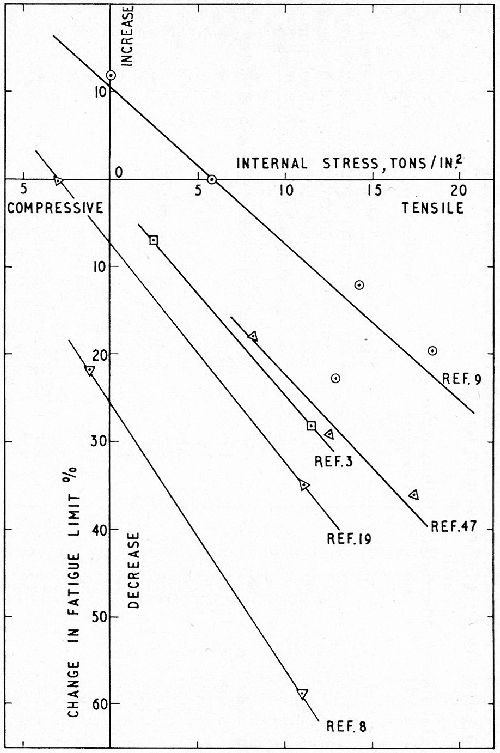
Figure 3 - Effect of internal stress in nickel deposits on fatigue strength of steel showing linear relationship. (Upper graph, Hothersall & Gardam3 - also four other investigations.)
Strength of substrate
The only date known to the author for nickel deposits are those of Forsman and Lundin.10 These authors plated fatigue test pieces made from a variety of steels of different strengths with nickel deposits 0.8 mil thick from an unspecified solution. The results of the fatigue tests suggested that there is a linear relationship between the fatigue limit of the substrate and the percentage change in fatigue strength on plating.
That the same relationship exists for chromium deposits on steel is confirmed by many investigations. Thus Stareck, et al. (loc cit) as a result of a survey of published data reported that, after chromium plating from the conventional solution and baking, the fatigue strength was constant at around 30,000 psi irrespective of the strength of the steel substrate over a range of tensile strength from 86,000 to 211,000 psi implying a linear relationship.
This question was investigated specifically by Williams and Hammond11 who chromium plated Wöhler fatigue test pieces made from a variety of ferrous materials ranging from Armco iron (TS 21 tsi; fatigue limit 10.8 tsi) to a quenched and tempered alloy steel of 100 tsi TS (fatigue limit 47.5 tsi).
The results, plotted in Fig. 4, confirmed the linear relationship and showed that, with the weakest materials, chromium plating produced an increase in fatigue limit.
Since for many types of steel the relationship between the tensile strength, fatigue limit or hardness (DPH) is approximately linear, it follows that the change of fatigue limit as a result of plating is also linear with these properties (Fig. 5).
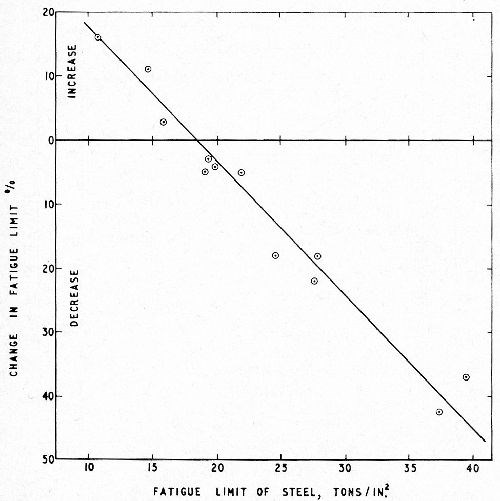
Figure 4 - Linear relationship between change in fatigue limit after chromium plating and the fatigue strength of the steel base; 6 mil chromium, internal stress 4 tsi.11
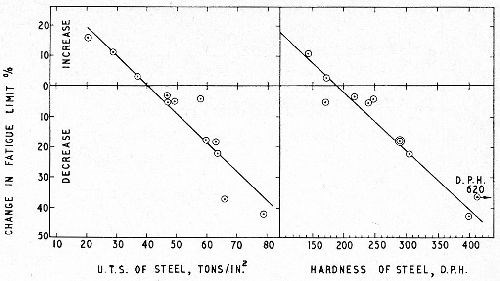
Figure 5 - Linear relationship between change in fatigue limit after chromium plating and the tensile strength of the steel base (left) and its hardness (right); 6 mil chromium, internal stress 4 tsi.11
As the change of fatigue limit with internal stress is also linear, it was possible from the experimental results to derive expressions from which the percentage change of fatigue limit on chromium plating |can be calculated from the known data viz:
L = 50 - 2Fs - 3S (1)
or L = 50 - T - 3S (2)
or L = 50 - (H/5) - 3S (3)
where L = change in fatigue limit (%)
Fs = fatigue limit of steel (tsi)
S = internal stress in deposit (tsi)
T = tensile strength of steel (tsi)
H = hardness of steel (DPH)
Closely similar expressions were derived by Stareck, et al. (loc cit) who give also an equation for baked deposits. Substitution of appropriate values in the above equations reveals the relatively unimportant influence of the stress factor compared with the strength factor, especially for high strength steels.
Effect of thickness of deposit
Chromium
An analysis of the literature by Williams and Hammond12 provided good grounds for concluding that, for both conventional and proprietary chromium solutions, the change in fatigue strength of steels of strengths ranging from 45 to 97 tsi is independent of thickness of the deposit for thicknesses between 1 and 17 mil, provided that the deposit is unbaked. This conclusion was also supported by the results of an investigation by the same authors6 based upon tests on two steels of 60 and 80 tsi tensile strength, plated from the conventional bath to thicknesses of 1, 6 and 12 mil. However, Sinnott,13 in fatigue tests of 1 and 10 mil deposits from various chromium solutions, though confirming this conclusion for "standard" and SRHS Cr110 solutions, found a thickness dependency for two other proprietary solutions.
Hard chromium plated components are, in general, baked to relieve hydrogen embrittlement however and the behavior of baked deposits is therefore of greater importance. In this connection, it has been found that, after baking, the fatigue strength loss is strongly dependent upon thickness. This is illustrated in Fig. 6 which shows a markedly increased loss for deposits 6 and 12 mil thick especially when baked in the temperature range 100 to 300°C (212 to 572°F). As shown later, however, this temperature range should be avoided for baking components subjected to critical fatigue stresses in service and, as is evident from the graphs, the thickness dependency largely disappears at high baking temperatures.
Nickel
Few investigators appear to have studied the effect of thickness specifically. However Barklie and Davies (loc cit), Hothersall and Gardam (loc cit) and Gadd14 all found that the percentage loss of fatigue strength increased with the thickness of deposit. This is not surprising, since, as shown by Brenner (loc cit) the inherent fatigue strength of nickel is maximum at the starting surface and diminishes as deposition proceeds owing to progressive softening (i.e., weakening) of the deposit.
From the practical standpoint, this effect is probably of little significance since nickel deposits used for engineering purposes are commonly very thick and are finished by machining.
Effect of baking
Chromium
Until comparatively recently, baking after chromium plating at temperatures ranging from 150 to 200°C (300 to 400°F) was commonly specified for relieving hydrogen embrittlement of the steel arising from the plating process. However, it was shown by Wiegand and Scheinost15 that heating chromium plated steel at 250°C (482°F) markedly reduced its fatigue limit.
In the first systematic study of the effect of baking on the fatigue limit of chromium plated steel, Logan16 at the National Bureau of Standards, confirmed the results of the German investigators, viz., that the fatigue strength of steel, already much reduced by chromium plating, was still further diminished by baking in this temperature range. Logan also made the important observation that, by baking at higher temperatures, e.g., 400-440°C (752-824°F), marked restoration of the fatigue strength occurred, though for hardened steel (hardness Rockwell C 40) the fatigue limit was not restored to the unplated value. Similar results were reported by Wellinger and Keil,17 Cabbie18 and Stareck, et al. (loc cit).
Williams and Hammond6 repeated this work for conventional chromium deposits 1, 6 and 12 mil thick on a steel of tensile strength 60 tsi but extended the upper limit of the baking temperature range to 620°C (1148°F). Replicate series of stress-strips, plated in the same solution and under the same conditions to a thickness of 1 mil were baked at the temperatures used in the fatigue tests, to determine the effect of the baking on the residual stress in the chromium deposit. The results of the fatigue and stress tests are shown in Figs. 7 and 8 respectively (see also Fig. 6 for similar fatigue tests on a steel of 80 tsi tensile strength). It will be noted that, by baking at the higher temperatures, almost complete restoration of the fatigue strength was achieved for both steels and, in fact, by baking the weaker steel at 500°C (932°F) the unplated fatigue strength was exceeded.
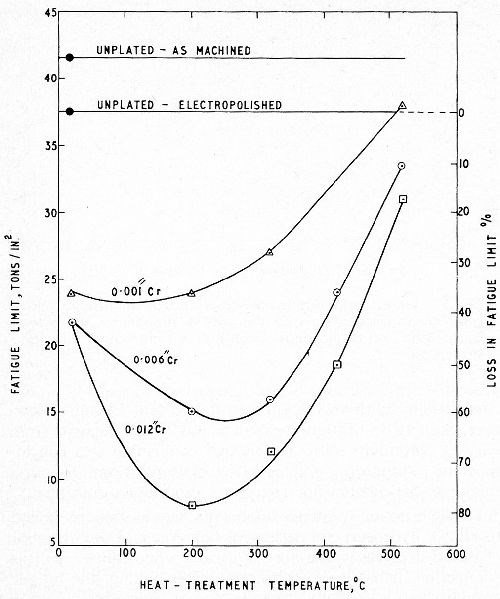
Figure 6 - Effect of baking on fatigue limit for three thicknesses of chromium; En 25 steel, DPH 400.6
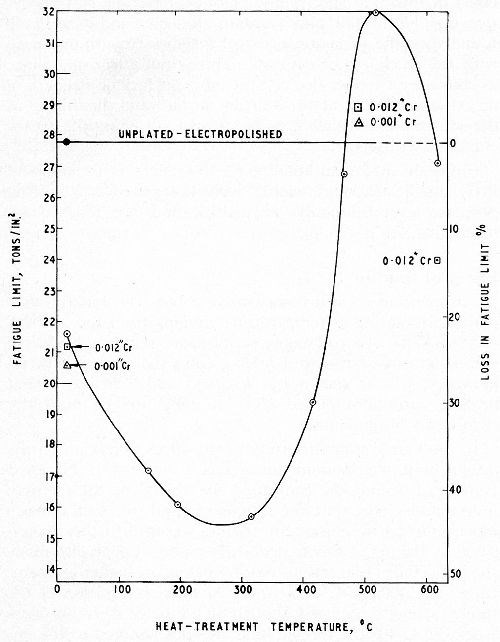
Figure 7 - Effect of baking on fatigue limit; En 25 steel DPH 305; 6 mil chromium.6
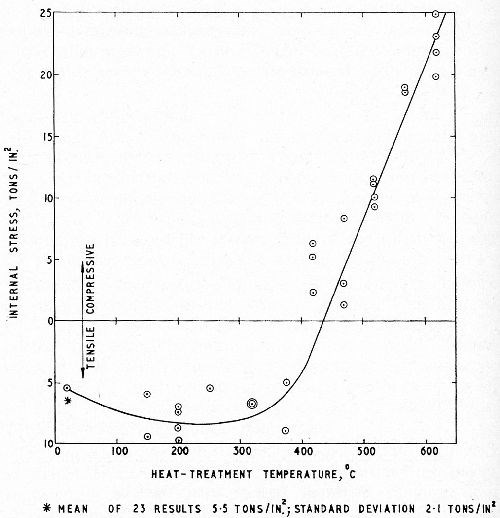
Figure 8 - Effect of baking on residual tensile stress; 1 mil chromium on steel strip.6
Comparison of the residual stress curve (Fig. 8) with the fatigue curves (Figs. 6 and 7) reflects the close connection between internal stress and the percentage change in fatigue strength. Figure 8 also provides evidence on which a theory on the mechanism of baking chromium plated steel can be based. Baking in the low temperature range, by inducing the well-known permanent contraction of electrodeposited chromium, increases the tensile stress in the deposit and so diminishes the fatigue strength of the plated steel. At higher baking temperatures (400°C and above), complete stress relief occurs. On cooling to normal temperature the chromium assumes compressive stress to an extent dependent upon the maximum temperature attained during baking. This is because of differential contraction between the chromium and the steel (the coefficient of expansion of chromium being approximately half that of steel). It is seen that very high compressive stresses may be induced in this way, and I believe that this accounts for the high fatigue strength achieved by high temperature baking.
The important practical conclusion emerges that, when maximum fatigue strength of a chromium plated component is an essential requirement, baking in the range 150 to 300°C (302 to 572°F) should be avoided. In fact, if high temperature baking is not practicable, it would be better not to bake at all provided the nature of the steel were such that no risk of hydrogen embrittlement would result.
Nickel
Heat-treatment sometimes improves the adhesion of nickel coatings and post-plating baking of heavy nickel deposits is frequently called for (e.g., U.K. Specification DTD 905 and U.S. Specification ASTM B242-54).
I am unaware of any investigation designed specifically to test the effect of such baking on the fatigue strength of nickel plated steel. However, the baking schedules are quite mild (e.g., 1 to 2 hours at 130 to 200°C (266 to 392°F)) and, apart from a possible slight reduction in tensile stress in the nickel (which would be advantageous), it seems unlikely that the fatigue strength would be affected either way. Thus Brenner, et al. found that the mechanical properties and structure of electrodeposited nickel were not significantly affected by baking below 200°C (392°F).
The effect of low temperature baking on specialized nickel deposits, e.g., those prepared from solutions containing organic addition agents, is less certain and this might profitably form the subject of an investigation.
Remedial measures
It follows from the mechanical considerations outlined above in connection with the mechanism of fatigue failure of plated metals, that practical measures to defeat loss of fatigue strength must depend upon either countering the effect of tensile stress (preferably replacing it by compressive stress - if not in the coating then in the surface layers of the substrate) or upon increasing the inherent fatigue strength of the coating. Ideally both results should be achieved simultaneously. It will be clear from the following outline of remedial measures, both for nickel or chromium that they all, in fact, depend upon these effects.
Chromium
For chromium deposits on steel there are two well established methods of combatting the loss of fatigue strength on plating, viz high temperature baking (440 to 480°C) or shot peening the steel prior to plating. In the first case, compressive stress is induced in the chromium thermally and in the latter on the surface of the substrate mechanically. The first method is obviously confined to those steels which will sustain baking at this temperature without loss of temper, but this includes a wide range of steels in the low or medium strength range (strength 80 tsi or less) and the upper limit of the range may soon be extended by the introduction of new high strength steels with high tempering temperatures. Another limitation of the baking method arises from the fact that electrodeposited chromium suffers some loss of hardness when baked at these temperatures (Table 4). Thus baking at 440°C (824°F) will reduce the hardness to approximately 650 DPH but this should be high enough for many applications in which maximum hardness is not the primary requirement, e.g., for corrosion protection combined with reasonably high wear resistance.
Table 4 - Effect of heat treatment for 2 to 6 hours on the hardness of electrodeposited chromium (from UK specification DTD 916A).

When the tempering temperature of the steel precludes the use of high temperature baking, or when it is essential to retain maximum hardness of the chromium, recourse may be had to shot-peening (or when the shape of the component is suitable, to rolling - see Ref. 4). These surface treatments induce a high degree of compressive stress in the steel surface which prevents fatigue cracks in the coating from propagating into the substrate. Williams and Hammond4 determined the stress distribution in standard Almen strips, peened to various intensities, by progressive anodic polishing and, from the results, the compressive stress 2 mil below the surface was calculated. At intensities of 0.010 A2 and upwards, the compressive stress exceeded 40 tsi (Fig. 9 - lowest curve). The upper curves show the influence of peening intensity on the fatigue limits of steels of two strengths, plated with 6 mil of chromium from the conventional bath. The peening intensities required to restore the fatigue limits of the chromium plated En 25 and SAE 4340 steels to their original (unpeened) values were approximately 0.012 and 0.015 A2, respectively.
Almen19 demonstrated the effectiveness of shot-peening for preventing loss of fatigue strength over ten years ago, and Fig. 10 shows the results he obtained with both nickel and chromium plating a steel base of approximately 50 tsi tensile strength. More recent investigations4,20 have shown that the method is equally effective for steels of 240,000 psi tensile strength or even higher.
To summarize the more important conclusions from these investigations it may be stated that:
- The loss of fatigue strength on chromium plating steels up to at least 240,000 psi tensile strength may be completely eliminated by shot-peening (or rolling) the steel before plating.
- To achieve this result, the peening intensity must be matched to the strength of the steel under treatment. Minimum intensities of 0.012 A2 or 0.015 A2 are necessary for steels of 180,000 and 224,000 psi, respectively.
- The peened and chromium plated steel may be baked at 200°C (392°F) to relieve hydrogen embrittlement without loss of fatigue strength.
- The roughening of the surface induced by peening may be eliminated by controlled grinding either of the steel before chromium plating (steel removed >1.5 to 2 mil), or of the chromium after plating.4
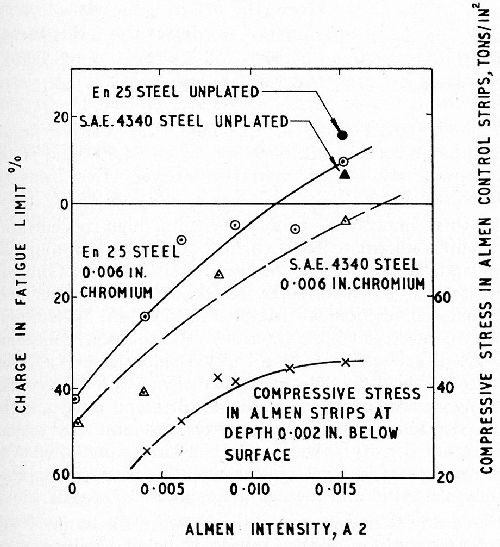
Figure 9 - Effect of shot-peening before chromium plating on the fatigue limit of En 25 (180,000 psi TS) and SAE 4340 (224,000 psi TS) steels. The lowest curve shows the calculated internal compressive stress 2 mil below the surface of Almen strips peened at various intensities.4
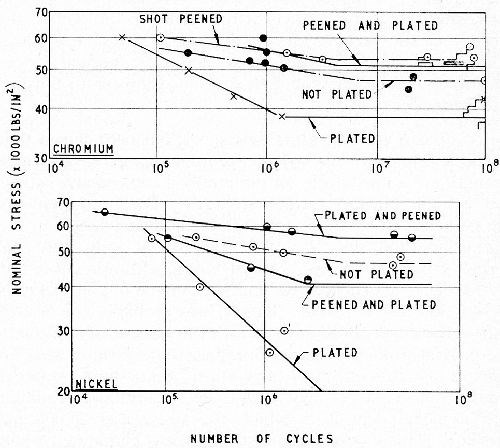
Figure 10 - Effect of shot-peening before or after chromium plating (top), or nickel plating (bottom) on the fatigue strength of steel rotating beam specimens.13
Nickel
The available processes for nickel deposition offer far more scope for controlling the internal stress and the mechanical properties of the deposit than is the case for chromium deposits. Thus, it is possible to control loss of fatigue strength on nickel plating either by shot-peening or by modifications to the bath formulation and operating conditions.
(a) Peening. As nickel from "straight" solutions readily acquires compressive internal stress on peening it is possible to peen the component either before or after nickel plating. Almen (loc cit) published results showing the results of fatigue tests by both methods (Fig. 10) and it is seen that he obtained a substantially higher fatigue limit by peening after plating. However, in heavy nickel applications, it is frequently necessary to remove a considerable thickness of the deposit by machining and this clearly involves a risk of removing the compressively stressed layer on which the efficacy of the peening process depends. For such applications therefore, either the steel should be peened before plating or the nickel should be machined almost to finished size before peening.
(b) Control via the plating process. It has been shown that desirable properties in a coating for minimizing loss of fatigue strength are a low tensile (or preferably a compressive) stress and a high inherent fatigue strength. Both requirements can be achieved by the use of suitable addition agents in the plating solution, since by such means, not only may the normal tensile stress be rendered strongly compressive, but the hardness and hence the tensile strength of the nickel is thereby increased. Almen (loc cit) was probably the first to suggest the use of addition agents for the purpose of improving the fatigue properties. He describes some fatigue tests in which a low strength steel (45,000 psi fatigue limit) suffered a reduction of 35 per cent in fatigue strength when plated with a nickel deposit in which the stress was 25,000 psi (tensile). This loss of fatigue strength was eliminated when the same steel was plated with nickel compressively stressed at 25,000 psi (Fig. 11). Scott21 found that nickel plating a hardenable stainless steel of 65 to 80 tsi TS in a sulfamate solution controlled to give a compressive stress of 3.3 tsi resulted in a loss of fatigue strength of only 1.3 per cent.
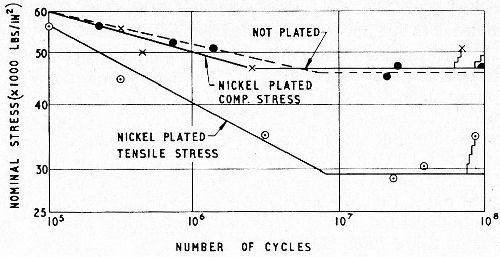
Figure 11 - Effect of nickel plating with controlled compressive stress on the fatigue strength of steel.19
On the other hand, Stareck, et al. (loc cit) found that plating a steel 100 tsi TS in a bright-nickel bath which produced a compressive stress of 1.34 tsi merely reduced the fall in fatigue limit from 59 to 22 per cent.
It is apparent, therefore, that the effectiveness of compressively stressed nickel deposits depends upon the intensity of the stress and upon the relative strengths of the deposit and of the basis metal.
Owing to the difficulty of controlling addition agents, the possibility of using "straight" solutions is attractive and the advantage of low-stress solutions such as the sulfamate bath for minimizing loss of fatigue strength has often been claimed. However, the mere reduction of tensile stress to a low value is not sufficient as was shown in tests by Fanner and Hammond22 in which Wöhler test-pieces of a steel 180,000 psi tensile strength were plated to a thickness of 6 mil in Watts and low-chloride sulfamate solutions giving internal stress values of 11,000 and 3,600 psi, respectively, and fatigue tested. The results showed only a marginal improvement viz. from 63 to 58 per cent loss in fatigue strength (Fig. 12).
The mechanical properties of the two types of nickel were almost identical:

It is apparent that, in this case, the relative fatigue strengths of deposit and basis metal (ca 34,000 and 90,000 psi respectively), rather than the internal stress, was the dominating factor.
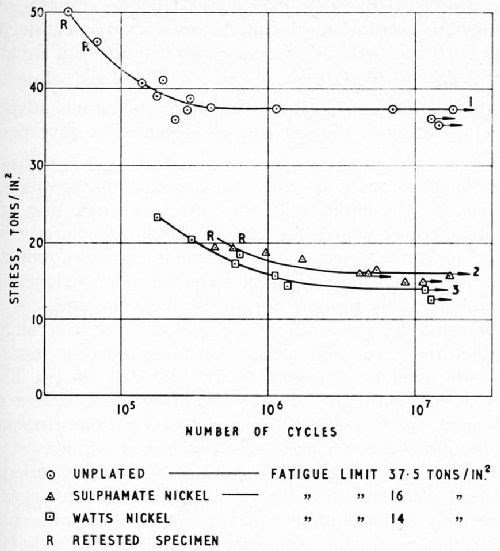
Figure 12 - Effect of nickel plating (6 mil deposits) on the fatigue strength of En 25 steel (180,000 psi TS); comparison of Watts solution (curve 3) with low-chloride sulfamate solution (curve 2); unplated steel (curve 1).22
In this connection, some work on a high-concentration "straight" sulfamate bath carried out in the Birmingham (England) laboratories of the International Nickel Company (Mond) Ltd. and reported by Kendrick23 at the Sixth International Conference on Metal Finishing in London last month is of considerable interest. The solution, containing 600 g/L nickel sulfamate, 5 g/L nickel chloride (6H2O), and 40 g/L boric acid, operates at high efficiency and at very high limiting current densities. The hardness and internal stress of the deposit may be controlled over wide limits by varying the current density. At the relatively low value of 50 A/ft2 the stress is 14,000 psi compressive, the hardness approximately 350 DPH and the ductility about 12 per cent.
Based on the linear relationship between the hardness and tensile strength of electrodeposited nickel established by Brenner (loc cit) this hardness would correspond to a tensile strength of approximately 150,000 psi and a fatigue strength of probably 75,000 psi, i.e., more than double the values for a normal straight Watts deposit. The properties of this new type of nickel deposit thus seem to be ideal for repair and salvaging work, and it is to be hoped that the work now published will be followed upon by mechanical tests and particularly fatigue tests.
SUSTAINED LOADING PROPERTIES
In this short survey of the effects of plating and ancillary processes on the mechanical properties of the basis material it is necessary to deal, however briefly, with the effect of hydrogen embrittlement. The subject was treated very fully at the Los Angeles Symposium of your Society in 1960, the Proceedings of which have been published24a,b and, in the time available, I can only touch upon the more important practical aspects.
Atomic hydrogen dissolves interstitially in certain metals or their alloys, notably in α iron and martensitic steels. Dissolved hydrogen has little effect on the mechanical properties of steel at applied stresses below the elastic limit, nor apparently does it affect the impact strength or the rotating-bend fatigue strength significantly. The tensile properties are little affected under rapid loading conditions as in the normal tensile test but, at low rates of strain, embrittlement is manifested by diminished elongation and reduction of area ("necking") although, in this case, fracture is accompanied by the plastic flow typical of ductile fractures.
However, from the practical point of view, the most important manifestation of hydrogen embrittlement is the delayed brittle fracture which may occur, possibly after several hundred hours, at applied stresses far below the normal strength of the steel (for example as low as 20 per cent).25,26
This type of failure is greatly accentuated by the presence of "notches" (for example sharp changes in cross-section of the component or surface imperfections) but it should be noted that, even in "un-notched" components, there are various structural features of the steel which, by acting as stress raisers, can promote fracture. Embrittlement of a given steel also depends on the overall concentration and, particularly, upon the distribution of hydrogen in the steel and, in contrast to the behavior of steels in the standard notched impact test, embrittlement due to hydrogen largely disappears at low temperatures. No completely satisfactory theory on the mechanism of hydrogen embrittlement in steel has yet been advanced but existing theories have been summarized by Hill.24a,b
Remedial measures
Steel being the major material for engineering construction and likely to remain so,† the problem of combating hydrogen embrittlement will stay with us and become more acute as the strength of steels in use increases. Its solution must depend upon a coordinated effort between the steel manufacturer, the Design Authority which specifies the material and the geometric form of the component, and the metal finishing specialist. While we are mainly concerned with the latter aspect, it is relevant to remark briefly on the earlier links in the chain.
The composition of the steel may have an important influence on its susceptibility to hydrogen embrittlement. Thus it has been stated that certain elements have a marked effect in diminishing its susceptibility. For example, Cotton27 found that steels of nominal AISI 4340 composition with 1.5 per cent Si were much less susceptible than the same steel with lower silicon contents. Klier, et al.28 found the same thing and these authors also suggest that embrittlement is minimized by low carbon content. Geyer, Lawless and Cohen29 in comparable embrittlement tests of cadmium plated SAE 4340 steel and a hot-work tool steel (SAE H- l1) of similar strengths, found a radical difference in the behavior of the two steels which they could account for only by a difference in their relative susceptibilities. It would appear that the development of a low-susceptibility high strength steel would constitute a very fruitful line of investigation. The steel maker could also help by keeping to low values the contents of sulfur, phosphorus and the initial hydrogen.
The designer's contribution it is suggested should be to take cognizance of the difficulties of preventing embrittlement during finishing processes by (1) avoiding the use of very high strength steels except where essential, (2) mitigating the effect of stress-raisers in the design by providing adequate radii and (3) calling for an adequate stress-relieving treatment before plating for such parts as may, by reason of their shape and method of manufacture, be liable to contain high tensile residual stresses. Such stresses may cause cracking or fracture even during processing and all high strength steel components should be given a pre-plating bake. The plater's difficulty will depend very largely on the tensile strength of the basis material. It is generally agreed that hydrogen embrittlement is no problem at strength levels below 180,000 psi (80 tsi), unless the material is very heavily cold-worked. Steels between 180,000 and 220,000 psi (say 80 to 100 tsi) are markedly susceptible and, at higher strength levels, the problems of devising a non-embrittling treatment become increasingly difficult. As at 1961, the U.S.A. Air Force did not permit cadmium plating of certain steels of strengths exceeding 220,000 psi.29
In metal finishing procedures any process that can liberate atomic hydrogen at the steel surface may lead to embrittlement; for example, acid pickling, cathodic cleaning or the electroplating process itself (since most plating processes operate at less than 100 per cent cathode efficiency). Even rinsing procedures are considered to be important.30
Remedial measures fall into two categories, namely those intended (1) to prevent or minimize hydrogen uptake during processing and (2) to remove the hydrogen after processing, by baking. Recommended procedures are commonly designed to incorporate both safeguards.
Minimizing hydrogen uptake
Cleaning and pickling
Cathodic alkaline cleaning is liable to cause embrittlement and should be replaced by soak or anodic cleaning. Acid pickling and dipping times should be restricted to the bare minimum and the temperature controlled (high temperatures favor embrittlement). Evidence on the effect of inhibitors in plain acid pickling is conflicting; some authors have claimed reduced embrittlement by their use but Read,31 referring to the mill-processing pickling of martensitic stainless steels, states that numerous investigations have shown that the occlusion of hydrogen may be greatly increased by the presence of inhibitors. Though shorter times are involved, it would be expected that the same would apply to pre-plating pickles. The purity of the pickling acid and possibly also of the plating solution seems likely to be important. Thus certain elements added to 10 per cent sulfuric acid, notably As, Se, Te, Sb and S compounds act as "promotors" favoring the adsorption of hydrogen in steel during cathodic charging.32a,b
When acid etching is necessary to secure adequate adhesion of the coating, anodic etching followed by rapid rinsing should be used. The high current density anodic etch in sulfuric acid as recommended by Gurklis, et al.33 for the cadmium plating of spring steel appears to be suitable for general application. For the treatment of very high strength steels, Hamilton and Levine34 recommend "electro-honing," that is, anodic polishing in mixed sulfuric-phosphoric acids (Battelle Electropolish Solution No. 2) optionally preceded by dry abrasive sandblasting (160-200 grits). Cash and Scheuerman35 have gone to the length of eliminating the etch altogether using dry abrasive blasting prior to cadmium plating and Lawrence (Ref. 30, Discussion) claims to obtain more satisfactory adhesion as well as reducing hydrogen occlusion by this means. The effectiveness of this approach was confirmed by the results of some work by a British aircraft manufacturer in which sustained loading tests were made on a three per cent Cr-Mo-V steel at 120 tsi tensile strength. At loads of and exceeding 70 per cent of the notched tensile strength, specimens cleaned by anodic etching and then cadmium plated and baked for 24 hours at 200°C (329°F) showed marginal embrittlement. No embrittlement was detected, however, when the anodic etching was replaced by a mild abrasive blasting treatment.36
Plating
While many plating processes when applied to high-strength steels involve a risk of hydrogen embrittlement, it is cadmium plating which presents the major industrial problem owing to its extensive use in the aircraft industry, and most of the published work relates to cadmium plating. Among the methods proposed for countering hydrogen embrittlement are the following:
- An obvious approach to the problem is to minimize hydrogen release by the use of plating baths with a high cathode efficiency and the employment of special high efficiency cyanide, fluoborate or sulfamate baths has been advocated for this purpose.†† However, an investigation of such solutions by Geyer, Lawless and Cohen29 based on sustained-loading tests on cadmium plated and baked SAE 4340 steel at 290,000 psi tensile strength throws considerable doubt on the efficacy of this method unless backed by other precautions. Thus brittle fracture occurred with plating efficiencies well over 99 per cent and the results appeared to be largely dependent on the structure of the coating as affected by the presence of addition agents. Porous deposits, by facilitating release of hydrogen during baking, can apparently compensate for a lower cathode efficiency and these authors suggested that the residual hydrogen embrittlement resulting from cadmium plating is a composite effect depending on both the cathode efficiency and the physical structure of the cadmium. In this connection, Cash and Scheuerman (loc cit) advocate a special cadmium plating process designed to give porous deposits.
- Another method aims to prevent co-deposition of hydrogen by the addition to the bath of an oxidizing agent (sodium nitrate) which acts as a cathodic depolarizer.34 A further addition is necessary to prevent excessive reduction of the nitrate, thereby stabilizing the cathode efficiency at a high value. This process appears to be very effective and was adopted for production use by an American aircraft manufacturer. In comparative sustained-loading tests of SAE 4340 steel heat-treated to 260,000-280,000 psi TS in which a conventional cadmium solution gave severe embrittlement, the "modified" process prevented embrittlement even though the test pieces were not baked. However, the modified process incorporates a number of other precautionary measures, including the use of a special low free-cyanide high-efficiency cadmium bath and special methods of preparation for plating, so that it is difficult to assess the individual contribution of the nitrate addition.
- Considerable success has been claimed for a Japanese process37a,b based on the addition of titanium to the plating bath. The cadmium deposit contains about 0.3 per cent of titanium and it has been suggested that the function of the titanium may be to neutralize the hydrogen by combining with it to form hydride. It is also claimed that this type of cadmium confers considerably higher corrosion protection.
- It is well known that the CN- ion catalyzes the adsorption of hydrogen in steel and, with a view to countering this effect Vlannes and Strauss have investigated alkaline cadmium plating baths complexed either with glycerine or with triethanolamine with promising results.38
- Since hydrogen release in plating processes derives from the electrolytic decomposition of water, attempts have been made to develop cadmium plating baths based on organic solvents and, as a step in this direction, Vlannes and Strauss have studied, with promising results, a solution of cadmium acetate dihydrate in methanol with or without a triethanolamine addition.39
- Yet another approach has been to prevent ingress of the hydrogen into the steel by the use of barrier undercoats. Beck and Jankowsky40 tried pyrophosphate copper and Watts-type bright nickel for this purpose using bend ductility, tensile ductility or sustained-loading tests to evaluate embrittlement. Provided the undercoats were thick enough, they found a marked reduction in embrittlement by the first two methods of testing. However, the beneficial effect of the undercoat was much less pronounced in the more sensitive sustained-loading test.
- A two-stage cadmium plating process, with an intermediate bake, was advocated by Johnson, Schneider and Troiano41 based on the theory that the thin cadmium undercoat, being porous, would readily permit release of hydrogen on baking but would act as a hydrogen barrier during the deposition of the second (thicker) cadmium coating. There seems to be some doubt in the U.K. as to the effectiveness of this method.
These brief notes serve only to illustrate the principles underlying a few of the more interesting procedures, but they suffice to indicate the wide scope of the efforts devoted to this problem in recent years.
Evaporated coatings
When dealing with methods of minimizing hydrogen uptake, reference must be made to the process of coating by vacuum evaporation which, by its nature, cannot involve co-deposition of hydrogen. Cadmium may readily be deposited by this process but low adhesion is normally a characteristic defect of evaporated metal coatings and its successful application to metal protection must depend upon solving this problem. This method of cadmium plating appears to have been studied much more extensively in the U.S.A. than in the U.K. and I understand that proprietary processes are available which are claimed to have overcome the adhesion difficulty.
Removing the hydrogen
In spite of all that has been said above concerning the efforts to minimize hydrogen uptake, the primary safeguard when dealing with "sensitive" steels is to bake after plating. (The importance of baking to stress relieve before plating has already been emphasized.) The concentration of hydrogen necessary to induce dangerous embrittlement under given conditions of stress is believed to vary according to the strength of the steel. Thus in a high or ultra-high strength steel very small concentrations of hydrogen may be sufficient to cause embrittlement whereas with weaker steels a much higher concentration may be tolerated and there is probably a critical concentration for any particular steel.
From the practical point of view therefore, the problem of counteracting embrittlement in the plated component consists in reducing the hydrogen content to a "safe" level. The only practicable method of achieving this is by baking. Baking operates in two ways: (1) by promoting diffusion of hydrogen into the bulk of the steel, thereby reducing the dangerous high concentrations produced near the surface during the pickling or plating processes and (2) by releasing the remainder of the hydrogen to the atmosphere. Both procedures are dependent upon time and temperature and both are facilitated by high temperatures and long times.
For all types of coating, an upper temperature limit is set by the tempering temperature of the steel and, in the case of surface-hardened or peened steels, by the need to retain the hardness or induced compressive stress. For cadmium plating, the low melting point of the metal (320°C) and the need to avoid oxidation or interdiffusion with the steel also limits the baking temperatures. For these reasons, typical specified de-embrittlement baking temperatures are usually in the range of 130 to 210°C.
Baking in vacuo does not materially assist the process of hydrogen release. The problem is accentuated for cadmium plating by the fact that the deposit is normally impermeable to hydrogen and this is particularly important when the entire surface of the component is plated. For this reason, the process previously referred to for depositing deliberately porous cadmium has obvious advantages and I understand that it is officially approved in the U.S.A. and is being used by several American aircraft manufacturers.
It is now appreciated that the baking times of one to three hours formerly used are insufficient, especially for cadmium plating on high strength steels and baking for 24 hours or more is now sometimes required. Post-plating de-embrittlement baking temperatures and times from various British Specifications are given in Table 5. Read has summarized and commented on various ASTM and U. S. Federal Specifications.
Table 5 - Post-plating baking temperatures and times from various British specifications.

CONCLUSION
This lecture, dealing almost entirely with the engineering limitations of electrodeposition has, by its nature, highlighted the defects and difficulties of the process. Little has been said about the special qualities of electrodeposits and the unique advantages of the process which, for over forty years, have enabled electrodeposition to play such an important part in industry and which, no doubt, will lead to a still greater contribution in the future. Even so it has been shown that the repair or hard-surfacing of components by the electrodeposition of nickel or chromium involves no significant loss in static strength and that in those special applications involving a fatigue hazard, well established methods for countering the limitations are available. In comparison, the phenomenon of hydrogen embrittlement is less understood, and, although for the majority of steels at present in use the problem can be overcome, it still imposes a bar to the use of the strongest classes of steel when these have to be protected or otherwise treated by electrodeposition. Much progress has been made in recent years however and, with the intense effort now being devoted to the problem, we may reasonably hope, that finishing techniques will keep pace, albeit a few years behind, with the development of stronger steels.
ACKNOWLEDGMENTS
British Crown copyright reserved in respect of Figs. 1 thru 9, and 12; reproduced with the permission of the Controller of Her Brittanic Majesty's Stationery Office. Figures 10, 11 - Courtesy of Product Finishing.
REFERENCES
1. H.L. Logan, J. Research Nat. Bur. Standards, 46 (6), 472 (1951); http://nvlpubs.nist.gov/nistpubs/jres/46/jresv46n6p472_A1b.pdf (last accessed 04/06/2015).
2. A.W. Hothersall, Proc. Inst. Mech. Eng., 152, 8 (1945).
3. R.D.H. Barklie and H.J. Davies, Proc. Inst. Mech. Eng., 731, (1930).
4. C. Williams and R.A.F. Hammond, Tech. Proc. Amer. Electroplaters' Soc., 46, 195 (1959).
5. A. Brenner, P. Burkhead and C. Jennings, J. Research, Nat. Bur. Standards, 40 (1), 31-58 (1948); http://nvlpubs.nist.gov/nistpubs/jres/40/jresv40n1p31_A1b.pdf (last accessed 04/06/2015).
6. C. Williams and R.A.F. Hammond, Trans. Inst. Metal Finishing, 32, 85 (1955).
7. A. Brenner, V. Zentner and C.W. Jennings, Plating, 39, 865-927 (1952).
8. J.E. Stareck, E.J. Seyb and A.C. Tulumello, Tech. Proc. Amer. Electroplaters' Soc., 42, 129-136 (1955).
9. A.W. Hothersall and G.E. Gardam, unpublished Ministry of Supply Rep. (1940) and supplementary Rep.
10. G. Forsman and E. Lundin, Proceedings of the 1st World Metallurgical Congress, Cleveland, Ohio (Amer. Soc. Metals), 1951; p. 606.
11. C. Williams and R.A.F. Hammond, Trans. Inst. Metal Finishing, 34, 317 (1957).
12. R.A.F. Hammond and C. Williams, Metallurgical Reviews, 5 (18), 165 (1960).
13. M.J. Sinnott, Univ. Michigan, Project M931, 1951.
14. E. R. Gadd, Proceedings of the International Conference on Fatigue of Metals, Institute of Mechanical Engineers, London; Amer. Soc. Mechanical Engineers, New York, 1956; p. 658.
15. H. Wiegand and R. Scheinost, Z.V.d I, 83, 635 (1939).
16. H. Logan, J. Research, Nat. Bur. Standards, 43 (2), 101-112 (1949); http://nvlpubs.nist.gov/nistpubs/jres/43/jresv43n2p101_A1b.pdf (last accessed 04/07/2015).
17. K. Wellinger and E. Keil, Metalloberflache, 2 (11), 233-236 (1948).
18. G.M. Cabbie Jr., Metal Finishing, 51 (6), 106 (1953).
19. J.O. Almen, Product Engineering, 22 (3), 109 (1951).
20. B. Cohen, Tech. Proc. Amer. Electroplaters' Soc., 45, 33-36 (1958).
21. B.E. Scott, Metal Finishing, 58 (11), 48 (1960).
22. D.A. Fanner and R.A.F. Hammond, Trans. Inst. Metal Finishing, 36, 32-42 (1959).
23. R. J. Kendrick, Proceedings Sixth International Conference on Electrodeposition and Metal Finishing, London, 1964, Trans. Inst. Metal Finishing, 1964 (In press [as of original publication date]).
24. (a) H.J. Read, Tech. Proc. Amer. Electroplaters' Soc., 47, 110-165 (1960)(Discussion pp. 241-246).
(b) Hydrogen Embrittlement in Metal Finishing, Harold J. Read, Ed., Reinhold Publishing Corporation, New York; Chapman and Hall, London, 1961.
25. H.J. Johnson, J.G. Morlet and A. R. Troiano, Trans. AIME, 212, 528-535 (1958).
26. A.R. Troiano, Corrosion, 15 (4), 207-212 (1959).
27. W.L. Cotton, Plating, 47, 169 (1960).
28. E.P. Klier, B.B. Muvdi and G. Sachs, Wright Air Development Centre, Tech. Rep., 1957, (W.A.D.C. 56-395, Part III).
29. Ref. 24(b), Chap. 6.
30. S.C. Lawrence Jr., Ref. 24(b), Chap. 5, 104.
31. H.J. Read, Ref. 24(b), Chap. 9, 197.
32. (a) W. Baukloh and G. Zimmerman, G. Arch. Eisenhüttenw., 9, 459 (1936).
(b) C.A. Zapffe and C.L. Faust, Tech. Proc. Amer. Electroplaters' Soc., 28, 1-20 (1940); Discussion, pp. 21-25.
33. J.A. Gurklis, L.D. McGraw and C.L. Faust, Plating, 47, 1146-1154 (1960).
34. W.F. Hamilton and M. Levine, Ref. 24(b), Chap. 8.
35. D.J. Cash and W. Scheuerman, Metal Progress, 75 (2), 90-93 (1959).
36. Unpublished U. K. Govt. Report.
37. (a) T. Sachimachi, Japanese Patent Application, 1960 - 18260.
(b) Boeing Process Specification, BAC 5804.
38. P.N. Vlannes and S.W. Strauss, Plating, 46, 1046 and 1153 (1959).
39. P.N. Vlannes and S.W. Strauss, Plating, 47, 926-932 (1960).
40. W. Beck and E.J. Jankowsky, Ref. 24(b), Chap. 7.
41. H.H. Johnson, E.K. Schneider and A.R. Troiano, Iron Age, 182 (5), 47 (1958).
42. M.B. Diggin, Trans. Inst. Metal Finishing, 31, 243-256 (1954).
43. D.S. Carr, Plating, 43, 1422-1429 (1956).
44. W.A. Wesley and E.J. Roehl, Trans. Electrochem. Soc., 82 (1), 37-53 (1942).
45. E.J. Roehl, Monthly Rev., Amer. Electroplaters' Soc., 34 (10), 1129 (1947).
46. E.J. Roehl and W.A. Wesley, Plating, 37, 142-146, 171 (1950).
47. (Fig. 3) L.H. Curkin and R.W. Moeller, Plating, 41, 1154-1158 (1954).
Footnotes:
*It should be borne in mind that the data are based upon laboratory tests of test pieces, usually of the rotating bend (Wöhler) type and some caution should be exercised in applying the conclusions to the performance of plated components. Very few actual fatigue failures positively ascribable to electrodeposition processes are reported by large operators in this field and the results of laboratory work on specimens tested to fracture may tend to give an exaggerated impression of the hazards involved. Nevertheless it may be claimed, I think, that these investigations give a reliable indication of tendencies and provide, therefore, valuable guidance to engineering and design authorities.
** Throughout this paper, "tsi" represents long tons (2240 lb) per square inch.
***Brenner draws attention to the fact that this is considerably higher than the value for metallurgical nickel (about 0.34).
†Titanium, the only notable competitor in the field of high strength/low weight parts is also subject to hydrogen embrittlement and of a type more difficult to deal with in some respects than that of steel.
†† It seems probable that it is the cathode efficiency during the early stages of deposition which is important rather than the overall efficiency, especially for impermeable coatings - a point which does not appear to have been generally appreciated.
About the author

His services to the Crown were recognized in the New Year Honors List for 1958 by his appointment as an Officer of the Order of the British Empire which he received at the hands of Her Majesty the Queen.
In 1927 he joined the Institute of Metal Finishing in which he has been active in committee work since 1931, becoming the first chairman of the London Branch Committee in 1947 and president 1955-1957. He was chairman of the Technical Sub-Committee for the Sixth International Metal Finishing Conference held in London in May 1964.
He has visited the U.S.A. on three occasions prior to delivering the Blum Lecture on June 15, 1964 - in 1950 as secretary of the Metal Finishing Productivity Team, in 1953 on a Government Mission and in 1959 to present a paper at the Fifth International Conference in Detroit.
RELATED CONTENT
-
Gold and Silver Plating Basics
An overview of precious metal electroplating processes.
-
Masking for Surface Finishing
Masking is employed in most any metal finishing operation where only a specifically defined area of the surface of a part must be exposed to a process. Conversely, masking may be employed on a surface where treatment is either not required or must be avoided. This article covers the many aspects of masking for metal finishing, including applications, methods and the various types of masking employed.
-
Zinc Electroplating
Choosing the best process for your operation.


















