Understanding Corrosion and Salt Spray
How it’s produced, NSS testing and how to get the best results possible.
#basics
“Why did these parts fail at 96 hours? The last ones went for over 120 hours without any issues.”
I am sure these words have escaped your lips at least once in your career as an electroplater in some form or fashion after reading the most recent report from your neutral salt spray (NSS) chamber operator.
Featured Content
To be less confused and frustrated by the results the plater receives from any accelerated corrosion testing facility, you must possess a basic understanding of corrosion. What it is; how it is produced; what NSS testing is; how you can produce, handle and package a product to give you the best results possible; and how you can engage your NSS operator when the root cause for any part failure seems to be generating out of the test itself, and not from the parts or the plating process itself.
To prevent corrosion, it’s imperative to know that corrosion is the process by which a metal in a solid state—such as zinc metal (Zn⁰)—is chemically changed due to a loss of electrons, turning solid metal into something different, often the cation Zn⁺².
There are a number of different corrosion reactions and types, but the one we focus on here is called oxygen-concentration cell corrosion, because that is the one employed when NSS tests are conducted. For instance, if we were to measure the concentration of oxygen directly in the middle of a drop of water on a piece of steel, we would find that it is a lot less than the concentration of oxygen at the very edge of the drop.
The different concentrations of oxygen in this droplet set up a corrosion cell wherein the oxygen-deficient area in the middle of the drop becomes the anode (or the corroding area just like you might witness in a plating tank) corroding away the iron in the steel and also becoming more acidic. The exterior of the drop (the cathode, or the oxygen-rich area) becomes more alkaline, thus precipitating out iron hydroxide in the form of red rust. This is because the cations (or positively charged particles of iron) are reduced or brought to a neutral state on the surface of the steel with the transfer of electrons. Anything that restricts the access of oxygen to a metal surface can develop what are termed differential aeration cells. Examples of restrictions would include anything from dust particles to a simple plastic washer.

Test plates are hung in the chamber for testing. Photo courtesy of Q-Lab.
Neutral Salt Spray Testing
NSS testing uses the oxygen-concentration cell corrosion mechanism to accelerate corrosion for use in performance analysis on a variety of substrates and coatings. Adding sodium chloride (NaCl) at a concentration of 5 percent keeps the corroded metal ions in solution so that they can act as conductors to enhance the corrosive effect. Salt helps to extend the life of each corrosion cell because it allows more metal to be in solution. The salt is actually increasing the solubility point of certain elements, like metal ions. Temperature is elevated during the NSS test as well in order to increase the speed of the electrochemical reactions attempting to take place. Parts are inclined to prevent the droplets of water being formed from becoming overly saturated. If the droplets fill up with metal ions and reach their saturation point, they will cease corroding the metal, which would defeat the whole purpose of the experiment.
A typical salt-spray chamber has some basic components: an air saturation tower that stabilizes the salt concentration, a reservoir for the solution itself, an atomization nozzle for the creation of the fog, supporting mechanisms to hold the parts, a method for distributing heat inside the chamber and a temperature controller.
If you have control over the way your parts are manufactured, you can do a lot to ensure the plating process runs smoothly and the coatings will be very corrosion resistant. For instance, an area of a part that has been stressed due to a crimping, machining or stamping operation is more likely to become corroded when compared with an area that has maintained its composition without significant stress.
Once the microscopic grain or micro-crystalline structure has been tampered with due to any type of force, a corrosive environment is poised to preferentially attack areas that have been modified, over regions that have sustained less deformation. Welds are a good example of this problem. Welders often use fluxes, or intermediate glues, which unfortunately can act as poultice—a moist conglomeration of conductive material contained in a soft, but self-contained slurry. The poultice is often composed of sodium, calcium, chloride, sulfate and other corrosive ionic agents that add to the conductivity of the electrolytic cell being formed.
Welds also tend to be porous, which means they can trap chemicals from the processing solutions causing bleed out and other phenomena, and they splatter metal fragments that can also negatively impact salt-spray results. Stress can also be created in the deposit itself by the inclusion of carbon, sulfur and other elements that are usually provided by proprietary additives like brighteners and carriers. The more stress your part or the deposit has, the more likely it is that those parts will become more anodic (more prone to corrosion) to their less-stressed counterparts.
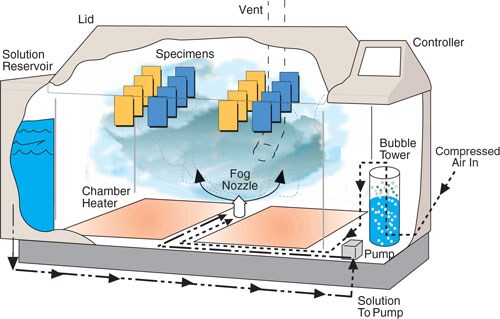
A typical salt-spray chamber has an air saturation tower that stabilizes the salt concentration, a reservoir for the solution itself, an atomization nozzle for the creation of the fog, supporting mechanisms to hold the parts, a method for distributing heat inside the chamber and a temperature controller. Photo courtesy of Q-Lab.
Designing Parts
Another factor to keep in mind is that part design has a lot to do with the ability of a part to be corrosion resistant, as do the substrate itself, the type of lubricants used, the finishing and manufacturing methods, and the overall geometric complexity of the piece. Simpler parts have a smaller or tighter range of current densities (few extreme high and low regions) and thus are able to be plated with more thickness uniformity and are thus more corrosion resistant. Parts that have deep recesses or more areas of low current density will tend to perform worse than parts that do not have recesses or crevices. Plating in these areas will be thinner, so the ability of a corrosive particle to penetrate to the base metal is greater.
If you cannot adjust the way the part is manufactured entirely, you may be able to convince the fabricators to fix a few elements that would positively affect deposit uniformity. Gently curving, convex surfaces are preferred over ones that have grooves, serrations, holes, concavities, fins, ribs, edges, valleys and recesses. Sharp angles and edges should be rounded off, softened, chamfered or beveled. Slightly convex shapes are actually preferred to flat areas. Grooves cut into a metal part should be rounded into a shallow, U-shape versus a sharp, deep, V-shape.
Try to avoid processing parts separately that will eventually need to be fitted together because the two parts together tend to form grooves that can produce a capillary that will soak up liquids during the plating process. If the manufacturer refuses to concede to making some alterations, you may have to chemically process the parts in a solution that simply has higher covering and throwing power to ensure higher thicknesses.
Packaging, Transporting
When packaging parts to be chamber tested, it is important to make sure the parts and packaging elements are free of contaminants, that they carry a preservative like a desiccant, are wrapped and cushioned to prevent mechanical damage, are compartmentalized to mitigate and moderate shock and vibration, and are not bulk packaged.
Other items to avoid when packaging parts are: cardboard, paper, and rubber (because of their sulfur components), flexible PVC (chlorine leaching is a potential hazard), any metal (because of the potential for galvanic cell formation), wood (due to the potential for resin leaks), and Ziploc bags. Permitted materials include: corrosion-safe paper products, polyethylene, polypropylene, cellophane, Formica, Styrofoam, fiberglass, and hard PVC.
The following information is not an exhaustive representation of everything that is required of an operator to perform his or her duties in running a proper salt-spray chamber, but rather it is a collection of information often missed when operators or customers analyze a chamber and its operation. Results from a salt-spray chamber can be affected by many things. Before submitting parts to a salt-spray chamber facility, ensure that the standards it employs fulfill the basics of what is expected from ASTM B117.
Sometimes the required number of collection vessels/funnels in the chamber is not followed. One funnel is to be positioned as close to the atomization nozzle as the nearest sample and as far away as the furthest sample. Verify the records your operator keeps on the salt solution’s specific gravity with their collection rate. The collection rate itself has a high amount of variability, requiring between 1-2 milliliters per hour.
Specimen are not allowed to touch the wall of the chamber as condensed salt solution will run down and flush the test specimen. Parts should not be shading one another from the spray or fog, nor should they be dripping onto each other. It would be prudent to ask that your chamber operator take a picture of the chamber with your part in it so that you can take note of your part’s orientation, its proximity to other parts in the chamber, and any other details that seem abnormal or disconcerting to you.
Keep in mind that corrosion areas should only be evaluated on the areas of the part that are at the correct angle, which is 15-30 degrees. A lot of customers will assume that every aspect of the part is under diagnosis, but this is not the case. For instance, threads are typically disregarded in salt-spray testing as they tend to accumulate and hold salt water. Corrosion runs, areas where a single area of corrosion seems to spread to the rest of the part, are to be ignored as well.
Monthly evaluations of the chamber’s corrosivity gives marked assurance that the chamber is running accurately and consistently. Steel panels are placed in the chamber, one nearest the collector and one farthest away. Panels are exposed for a determined number of hours that correspond with a range of weight loss. For instance, panels exposed for 96 hours should average a weight loss of 1.5347 grams with a variance of +/- 21 percent. What this means is that the same part, with the same thickness, the same type of deposit, and the same coating, is allowed to vary by 21 percent in either direction for each trial run in the same chamber and still be considered a well-run chamber.
In other words, if you wanted some chromated zinc-plated parts to pass 96 hours in salt spray to first white rust (FWW), the exact same part, with the exact same chromate thickness, is expected to reach anywhere between 75 and 116 hours in a good salt-spray chamber. Therefore, unless your part averages 122 hours in salt spray, on average, half your parts will fail to reach 96 hours and the other half will reach 96 hours and above. The authorized or permitted variability when testing parts in different chambers for the chamber to be considered good or accurate is plus or minus 36 percent. If you wanted some chromated zinc-plated parts to pass 96 hours in salt spray to white rust, the exact same part is expected to reach anywhere between 61 and 131 hours if placed in two different chambers. So, unless your part averages 150 hours in salt spray, on average, half your parts will fail and half your parts will get to 96 hours and above.
Corrosion Resistance
There are a number of variables involved not only in conditioning a surface to be corrosion resistant to a particular standard, but also in making sure that the test is done correctly and no other elements outside the finishing purview are the cause of failures.
The key is in learning more about how to design, plate, coat, package and transport parts and how to engage your NSS operator to ensure he is operating his chamber with every degree of accuracy and repeatability.
Adam Blakeley is a CEF 1 and a technical service representative for MacDermid Enthone. For information, visit macdermid.com. For information on H.E. Orr Co., visit heorr.com. For info on G2MT Labs, visit g2mtlabs.com. Some information for this article came from Frank Altmayer’s NASF corrosion course.
Originally published in the January 2016 issue.
RELATED CONTENT
-
Fixing Corrosion Between Anodized Aluminum and Steel
Anne Deacon Juhl, Ph.D., with AluConsult, says Galvanic corrosion is due to an electrical contact with a more noble metal or a nonmetallic conductor in a conductive environment.
-
What is Electrocoating?
E-coat can produce uniform finishes with excellent coverage and outstanding corrosion resistance.
-
Nickel Electroplating
Applications, plating solutions, brighteners, good operating practices and troubleshooting.


















