Nanostructure of the Anodic and Nanomaterials Nanoparticles in Polyelectrolyte Multilayer-by-Layer (LbL) Films
Microstructure and elemental characterizations have indicated that the finish of a coating with a Layer-by-Layer (LbL) film results in a closely multilayered coating with a smoother surface. In this paper, the principles of assembly are discussed together with the properties of nanoparticles and LbL polymeric assembly essential in building hybrid coatings.
#nasf #additive-manufacturing #surfin
by
Xavier Albort Ventura*
Laboratory Electrochemical R&D, Barcelona, Spain
University Politecnic of Catalonia, Barcelona, Spain
Tecnocrom Industrial Cabrera de Mar, Barcelona, Spain
Editor’s Note: A printable pdf of this paper can be accessed and printed HERE
Featured Content
ABSTRACT
Microstructure and elemental characterizations have indicated that the finish of a coating with a Layer-by-Layer (LbL) film results in a closely multilayered coating with a smoother surface. In this paper, the principles of assembly are discussed together with the properties of nanoparticles and LbL polymeric assembly essential in building hybrid coatings. Such coatings represent the key components in enhancing mechanical properties, enabling activation by laser light or ultrasound, constructing anisotropic and multicompartment structures and facilitating the development of novel sensors and movable particles. Here, we discuss the increasingly important role of inorganic nanoparticles in the Layer-by-Layer assembly, effectively leading to the construction of the so-called hybrid coatings. Applications and emerging trends in the development of such novel materials are also identified.
Key Words and phrases: multilayer, corrosion, impedance, Layer-by-Layer, nanoparticles, polymers, films, LbL coatings, polyelectrolyte multilayers, the properties of nanoparticles and Layer-by Layer polymeric assembly essential in building hybrid coatings, the binary pore, Layer-by-Layer films
1. Introduction
The design and fabrication of soft nano/micro-sized assemblies for encapsulation and controlled release of active compounds has been a very active field in the past few decades. The extensive development of this field has been due to its important implications in many areas of science and technology, from functional foods to pest control and from cosmetics to drug delivery.
The growing interest in the application of supramolecular functional materials in such applications has led to the development of different techniques for the self-assembly of materials in a controlled manner. Building blocks can be chosen so that they have the appropriate properties and structures to fulfill the requirements associated with different applications.
Different methods have been developed for manufacturing materials with tunable composition, structure and shape. The Layer-by-Layer (LbL) technique is one of the more noteworthy due to its extraordinary simplicity and versatility.1 The LbL method initially consisted of the alternate deposition of oppositely charged materials, mainly polyelectrolytes onto flat macroscopic solid charged substrates through electrostatic interactions.2,3 The LbL method can be considered as a template-assisted methodology, which needs a precursor structure, sacrificial or not, to build the final supramolecular material.
Beyond the use of macroscopic flat solid substrates, other types of substrates with multiple chemical natures, shapes and sizes are frequently used as templates, including colloidal particles,4 liposomes or vesicles5 and fluid interfaces.6
In addition to the classical LbL assembly mediated through electrostatic interactions, it is also possible to build materials by the LbL method taking advantage of other types of interactions including hydrogen bonds, chemical bonds, host- guest interactions or acid-base reaction.7-9
This has expanded the range of materials used as building blocks, beyond simple polyelectrolytes. Currently, the list of potential materials includes different chemical compounds, and nano-and micro-objects, ranging from simple molecules to carbon nanotubes or nano- or micro-particles, as well as from synthetic polymers to different types of biomolecules (nucleic acids, proteins, peptides or lipids).8,10-17
The final structure of the materials-built ranges from particles with onion-like structures to sponges, membranes or nanotubes,18 which offer many possibilities for the use of LbL materials as platforms for the encapsulation and controlled delivery of active compounds.19-20 Furthermore, the LbL method provides the bases to tailor the optical, electrical, mechanical and many other physico-chemical properties of the fabricated materials.
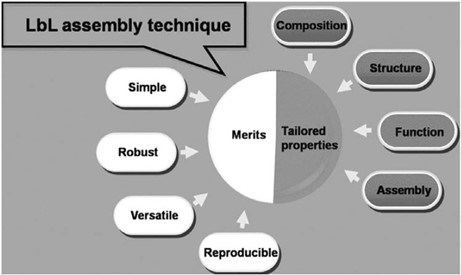
Figure 1 - Main advantages and characteristics of the LbL approach on the fabrication of functional materials. Reproduced from Ref. 21, Copyright (2016), with permission from the Royal Society of Chemistry.
In addition to the variables mentioned above, there are many other variables having an important impact on the fabrication of functional materials, including charge density of the molecules, concentration, ionic strength and the type of ions, solvent quality for the molecules, pH and temperature.22-28 The precise understanding of their role is essential for the fabrication of supramolecular materials with controlled and tunable structures and physico-chemical properties.
The knowledge of the effect of these variables is important for determining the properties of any material. In the case of the LbL films however, their control is even more important. This review intends to afford a complete overview of some of the most relevant physico-chemical aspects related to the building of LbL films, paying particular attention to the aspects relevant to the fabrication of functional materials with applications as platform for the encapsulation and controlled release of active molecules.
The advantages and disadvantages of using modified sol-gel polymer films and hybrid system coatings will also be discussed, as well as the methodologies for the chemical characterization and the feasibility of evaluating the mechanical properties of the coatings.
The microstructure and elemental characterization indicate that the finish of the coating with the LbL film results in a closely connected multilayered coating with a smoother surface. The principles of assembly are discussed together with the proprieties of nanoparticles and Layer-by-Layer polymeric assembly essential in building hybrid coatings
2. Layer-by-layer assembly methodology
The assembly methodologies used for the fabrication of Layer-by-Layer materials have not changed so much since the seminal works. Only slight modifications due to particular characteristics of the templates have been introduced.
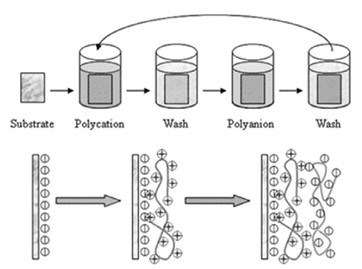
Figure 2 – Schematic diagram of the polyelectrolyte LbL deposition using a common dipping methodology. Reproduced from Ref. 29, Copyright (2012), with permission from the Royal Society of Chemistry.
Figure 2 shows the alternate dipping of flat macroscopic substrates into solutions including intermediate rinsing cycles for removing the material not strongly absorbed. This is most necessary in the case of polyelectrolytes, because undesirable interpolyelectrolyte complexes can precipitate, thus modifying the composition structure and properties of the final material.29
Figure 3 shows two more methods for the deposition of Layer-by-Layer films onto flat macroscopic templates: spin coating and spraying.30
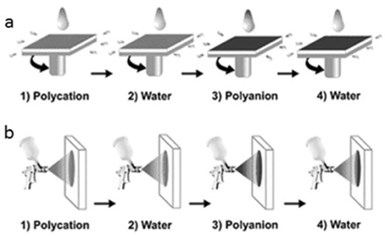
Figure 3 - Schematic diagram of the polyelectrolyte LbL deposition using (a) spin coating and (b) spraying. Reproduced from Ref. 30, Copyright (2012), with permission from the Royal Society of Chemistry.
They will not be discussed in this review because they can only be used for flat substrates that have a limited use as cargo systems.
Most interesting from the practical point of view for fabrication on platforms for encapsulation and controlled release of active compounds is the use colloidal particles, vesicles and liposomes or micelles as templates for the Layer-by-Layer deposition.5,31
The classical approach is based on the alternate deposition of the polyelectrolytes onto the particle surface in aqueous media with intermediate cleaning and centrifugation steps in order to remove the excess of non-absorbed polyelectrolyte, thus avoiding the formation of interpolyelectrolyte complexes in the media during the addition of the subsequent opposite charged polyelectrolytes.32 Once the desired number of layers is reached, hollow capsules can be obtained by chemical dissolution of the core, using an appropriate agent that does not affect the integrity of the shell, while being able to dissolve the core. This treatment depends on the chemical nature of the template, and some classical methods used are (1) diluted hydrochloride acid for melamine formaldehyde resin particles, (2) fluoride acid for silicon oxide particles and (3) tetrahydrofuran when polystyrene is used as template.32
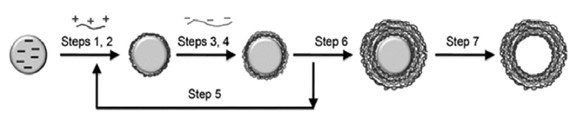
Figure 4 – Schematic diagram of the polyelectrolyte LbL deposition onto a colloidal microparticle: Steps 1 and 3 involve the adsorption of polyelectrolyte layers; Steps 2 and 4 refer to the cleaning process (centrifugation or other procedure); Steps 5 and 6 indicate the repetition of the sequence of the steps from 1 to 4 the number of times needed to reach the required number of layers, and Step 7 is the dissolution stage of the core to manufacture the hollow capsule. Reproduced from Ref. 32, Copyright (2011), with permission from the Royal Society of Chemistry.
Figure 4 shows a schematic diagram of the protocol most frequently used for the assembly of the polyelectrolyte onto colloidal templates. However, this method presents some problems associated with the fact that some polyelectrolyte remains in solution after each deposition cycle.
Another interesting alternative for scaling up the fabrication of LbL capsules is the use of microfluidic chips. However, its use is limited by the monitorization and optimization of the process for each case. Also, expensive instrumentation is necessary.
The growing interest in the application of LbL materials in fields such as drug delivery requires the use of biocompatible templates, which explains the attention received by liposomes and vesicles as templates in the last few years.5,33,34 These templates can be coated with other biocompatible polymers, which make them suitable for their use as cargo systems of both hydrophobic and hydrophilic actives solubilized in the lipid shell or in the inner region of the vesicle or liposome, respectively.
Furthermore, these systems can be easily functionalized for targeted delivery. Despite these advantages, the coating process of vesicles is trickier than that of conventional colloidal particles. This is due in part to the fact that the centrifugation step can lead to the fusion of vesicles or liposomes or to the aggregation of emulsion drops. Thus, centrifugation can be used for cleaning the dispersion only after the deposition of a certain number of coating layers that ensure the formation of a shell rigid enough to avoid these undesired effects
Figure 5 shows a schematic diagram of an LbL assembly protocol for the fabrication of capsules using liposomes or vesicles.34
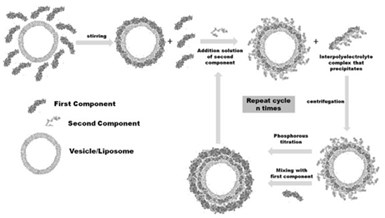
Figure 5 - Schematic representation of the assembly process of LbL films onto liposomes or vesicles. Reprinted from Ref. 34, Copyright (2014), with permission of Springer.
A dilute liposome/vesicle suspension is mixed with a solution of the compound forming the first layer. After the adsorption, the second component is added in excess to form the interpolyelectrolyte complex that precipitates and to form the second layer of the LbL film. Once the first bilayer is formed, the suspension of coated liposomes is mixed again with the first component to start the formation second bilayer.
The adsorption/separation steps are repeated the number of times needed until the desired number of layers is deposited. It is worth mentioning that around 8% of the total number of liposomes/vesicles is lost after each bilayer is deposited, which is an important limiting factor on the maximum number of layers. The maximum number of layers deposited onto vesicles/liposomes is generally not higher than 12, not a significant problem for capsules used for delivering active compounds.
It is worth mentioning that the above method for vesicles, liposomes and suspension droplets is limited to dilute solutions. This is an important drawback for the application of this type of system in the development of commercial products.35 Emulsions are particularly interesting systems because they allow the encapsulation of a higher number of lipophilic actives.
Figure 6 shows a schematic representation of the Layer-by-Layer approach used for the fabrication of this type of nano carrier. It is worth noting that LbL multilayers are generally highly hydrated systems, containing counterions. In addition, the adsorption of macromolecules onto the surface can be considered an irreversible process. These issues are very important for potential application of these systems because they confer enhanced stability to the multilayer in different environments.
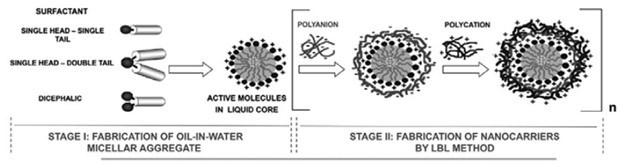
Figure 6 - Schematic diagram of the possibilities offered for the fabrication of nanocarriers using emulsions as templates. Reprinted from Ref. 35, Copyright (2015), with permission from Elsevier.
3. Electrostatic entropic factors: assembly driving forces
The degree of charge inversion strongly depends on the specific polyelectrolyte pair but shows no significant dependence on the assembly conditions (ionic strength, pH, etc.). The degree of over-compensation is maximum at the surface of the layers and decays exponentially towards the inner region of the multilayer.24,26
Despite the importance of charge overcompensation, it is possible to build LbL films using neutral species.9 This imposes a zero net charge condition for the multilayer stability, which can be considered as the underlying physico-chemical reason for the appearance of different compensation mechanisms in polyelectrolyte multilayers. Two different mechanisms can be found in LbL polyelectrolyte films to ensure their neutrality and are related to the different extension of the entropic factors in the assembly of the materials.
The first mechanism, the so-called intrinsic compensation for the charge compensation in LbL polyelectrolyte films, involves the complexation of oppositely charged polyelectrolyte in adjacent layers. Therefore, the intrinsic mechanism presents the highest degree of ionic cross-linking that can appear in LbL films. The formation of these complexes is associated with the release of a large number of counterions from the polymer material to the solution, thus highly increasing the entropy that leads to a significant decrease of free energy. In these films the entropic factors become the main driving force for the multilayer assembly.
On the other hand, when counterions remain trapped in the structure of the film, a second compensation mechanism appears, the so-called extrinsic one. In films presenting this type of compensation, the counterions are mandatory for the charge compensation and a broad range of stoichiometries is possible. In this case entropy, has a smaller effect. Figure 7 shows schematically both types of charge compensation mechanism.
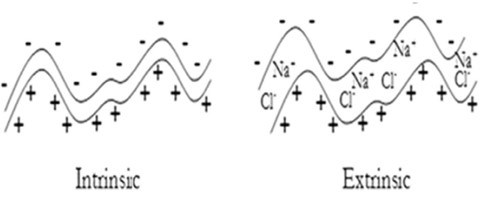
Figure 7 – Schematic diagrams of the two different types of charge compensation appearing in polyelectrolyte multilayers. Reproduced from Ref. 24, Copyright (2009), with permission from The Royal Society of Chemistry.
Most of the Layer-by-Layer, polyelectrolyte films have extrinsic compensation,8,11,12,24,26,36 the intrinsic one appearing mainly when highly charged polyelectrolytes are assembled. However, the compensation mechanism also depends on the assembly conditions.26
Precise control of the compensation mechanism is essential for controlling the structure and properties of the final material. Among the parameters influencing the compensation mechanism, the ionic strength of the solutions is probably the most important because of its effect on the charge density of the chains.24,25 The ionic strength can change the compensation mechanism from mainly intrinsic to extrinsic, explained in terms of the entropic effects on the assembly process. At low ionic strength, the counterions strongly increase the entropy, leading to primarily intrinsic compensation. At high ionic strengths, the ionic concentration in the bulk is high, and thus the release of more counterions does not significantly affect the entropy of the system. Therefore, counterions remain trapped in the polymer matrix as shown in Fig. 8.
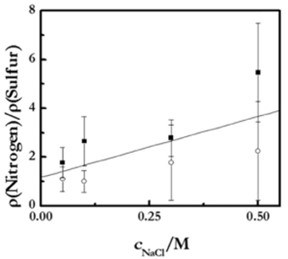
Figure 8 - Ratio between the surface densities of monomers, ρmonomer, containing nitrogen (PDADMAC) and sulphur (PSS) in (PDADMAC + PSS) in multilayers with different ionic strengths, as obtained by ellipsometry (■) and XPS (○). Notice that the different amount of adsorbed PDADMAC and PSS is strong evidence of the existence of extrinsic compensation. Reproduced from Ref. 25, under license Creative Common 2.0, with permission from Beilstein-Institute, Copyright (2016).
The figure shows the increase of the extrinsic compensations with the ionic strength for the poly (dialiimetylammonium chloride) PDADMAC, PSS, system as obtained by two independent techniques - ellipsometry and X-ray photoelectron spectroscopy (XPS). The increase of the ratio between the number of nitrogen and sulphur with the ionic strength is a good indication of the compensation mechanism. The formation of more swollen layers, with a higher ionic concentration, leads to increased roughness.24,25
The ionic equilibrium in Layer-by-Layer films can be modified by changing any parameter affecting the charge compensation mechanism such as the type of polyelectrolyte and their charge density, the pH and the solvent quality.11,12,37-39
Other variables that effect the entropic contributions of the assembly process40 are the release and reorientation of hydration water molecules, and the entropy penalty arising from the decrease of the degrees of freedom of the molecules due to their attachment to the surface. Despite this unfavorable effect, its role in is usually rather small, and can be neglected in most cases.
From a basic physico-chemical point of view, it is possible to assume that electrostatic control does not sufficiently explain the assembly of LbL films, particularly when low charge density materials are used, or when the assembly occurs under conditions in which the screening of the charge is too high. A clear indication is that it is possible to assemble multilayers using neutral polymers.9
4. Growth mechanisms of Layer-by-Layer materials
Beyond the different interactions involved in multilayer assembly, there is incomplete knowledge of different aspects related to the control of the assembly of LbL multilayers.
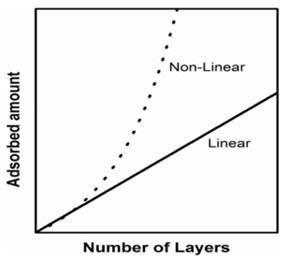
Figure 9 – Schematic diagram of the two common growth dependences on LbL films: linear vs. non- linear.
Among them, the origin of the appearance of two different types of dependence of the adsorbed amount on the number of layers, N, (growth mechanisms) in still under discussion. Two types of growth, linear and non-linear, frequently referred to as exponential, are the most frequently, found in literature,23,24,41 although more exotic dependences have been described in recent years.42 Figure 9 shows the schemes of the linear and non-linear dependencies.
Linear growth is characterized by the appearance of a quasi-linear dependence of the adsorbed amount on N, being typically found in (PAH+PSS) multilayers independent of assembly conditions, and for the (PDADMAC+PSS) system, when assembled under conditions in which a high charge density of the polymers, mainly PDADMAC, are guaranteed. In non-linear growth, the dependence of the adsorbed amount on N grows more rapidly than that expected for linear growth.
This mechanism is often considered to be exponential even though the dependence is not purely exponential, and it is observed in the (PDADMAC+PSS) system under conditions in which there is a reduced effective charge of the polymers under conditions of high ionic strength23,24,36 and in many biopolymer-based materials.43-45
There are several explanations to justify the appearance of different growth trends in Layer-by-Layer materials. The first considers that the appearance of two different growth mechanisms is associated with the in-and-out diffusion of at least one of the building blocks within the supramolecular architecture during the successive deposition cycles, leading to an increase of the adsorbed amount in the successive deposition cycles.46,47 This explanation has been long accepted as the main justification of the appearance of two different growth mechanisms. Even in recent years, a detailed analysis of the adsorption kinetics of the layers has evidenced that the interdiffusion is not limited to non-linear growth materials.23
The explanation based on the role of the interdiffusion considers the existence of a potential along the LbL material, associated with a charge excess due to the layer’s adsorption. Thus, the interdiffusion must take place until the equilibration of the potential is reached.47
An alternative explanation considers the differences in the multilayer roughness as the source for the appearance of two different growth dependences. For high roughness, the adsorption of successive layers involves a continuous increase of the area available for the adsorption, which leads to an increase of the adsorbed amount in the successive deposition cycles, and thus leading to non-linear growth.24,25,48
The models do not rule out the possible appearance of interdiffusion, which is the basis for the explanation of the transition from linear to non-linear growth in the most classical model. However, they do not limit the appearance of interdiffusion to one of the growth mechanisms.
5. Incorporation of nanoparticles in LbL coatings: Hardness enhancement and additional properties through inorganic building blocks
Although nanoparticles have been perhaps most frequently used for LbL functionalization, various nanostructured building blocks, i.e., nanocomposite films with nanoparticles embedded in the layered structure, have been shown to be a highly effective class of material to tune various properties (including mechanical stiffness) with a high degree of accuracy (Fig. 10).49
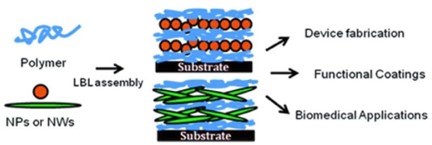
Figure 10 - Schematics of incorporated inorganic nanoparticles, nanowires and nanosheets in Layer-by-Layer assemblies. Reproduced with permission from Ref. 49, Copyright 2008 American Chemical Society.
Various methods have been used for LbL assembly with nanoparticles where, in addition to dipping or incubation, spin-spray LbL assembly, spin-coating, spray assisted alignments and cross-linking after infiltration50 were used. Nanocomposite LbL films containing immobilized ZnO/SiO2 nanoparticles were investigated to provide UV protection properties.51 Alternating layers of barium oxide nanoparticles/alginate were fabricated on top of beta cells via LbL, exhibiting robust antioxidant activity and providing excellent protection to these cells upon exposure 10-3M of H2O2 without affecting the metabolism of the cells.52
6. Planar Layer-by-Layer coatings and their functionalization by nanoparticles
Nanoparticles have been essential components of planar layers, where they were used for enhancement of mechanical properties, remote activation, controlling the stiffness of the coatings, for incorporating quantum dots and corrosion protection.
7. Enhancement of mechanical properties and remote activation of LbL coatings
Mechanical stability of capsules and films plays an essential role in enabling practical applications. The addition of nanoparticles would, thus increase the shell stiffness. It should be added that the same concept of strengthening mechanical properties of LbL, by adding nanoparticles, has been also shown for planar coatings. Regarding mechanical properties of planar coatings, mechanical properties of nanometer thin LbL films,53,54 (layer reported to be 1-2 nm) resemble those of the substrate on which they were assembled (often glass, metal or plastic). However, thicker LbL films55 with a high molecular dynamic of polyelectrolytes, are rather soft, hindering cell growth in biomedical applications. One way to increase the stiffness of the coatings is to use chemical cross-linking.56,57
Another possibility of improving the mechanical properties and enabling the adherence of cells on the coatings is to functionalize the surface with metal nanoparticles.58 Recently, particles and nanoparticles have been used to stimulate cell adhesion on the planar and soft hydrogel coatings relevant to osteoblasts, and different types of cells.59
8. Passive and active activation of Layer-by-Layer coatings
Passively active coatings are those in which they or some of their components exhibit a certain functionality. An active functionality of nanoparticles was to functionalize the micrometer thick LbL coatings with nanoparticles and use them as active absorption centers for remote laser action. Nanoparticle functionalization of such a soft LbL coating is depicted in Figs. 11 and 12.
However, this is not the only functionality of the nanoparticles in the coatings. They can be also used to control the masking for fabricating Janus particles, i.e., special types of nano- or microparticles whose surfaces have two or more distinct physical properties.
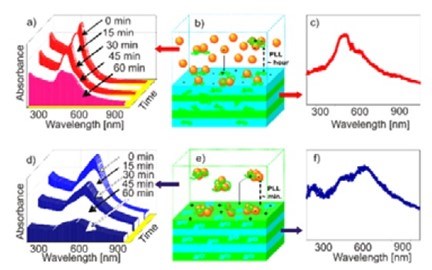
Figure 11 - (Left) Kinetics of adsorption of non-aggregated (a-c) and aggregated (d-f) nanoparticles onto biocompatible poly-L-lysine (PLL)/hyaluronic acid (HA) films. (Adsorption on (PLL/HA)24 films are shown in (a–c), while adsorption on (PLL/HA)24PLL films is demonstrated in (d–f)). Ultraviolet-visible (UV/Vis) absorption spectra of the supernatant solution during adsorption in (a) and (d) were recorded at 15 min time intervals. Schematics of the interaction of nanoparticles and the films in non-aggregated and aggregated states are demonstrated in (b) and (e), and the corresponding UV/Vis absorption spectra of the films after nanoparticle adsorption are given in (c) and (f), respectively. Reproduced with permission from Ref. 60. Copyright 2010, Wiley-VCH Verlag GmbH.
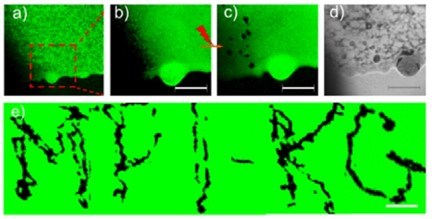
Figure 12 – (a) Confocal laser scanning microscope images of a (PLL/HA)24 film functionalized with aggregated gold nanoparticles. The enlarged area shown in (b) was exposed to a near-infrared (NIR) laser leaving characteristic dark spots (c). The dark spots (c) affected by the near-IR laser can be also seen in the transmission image (d). The scale bars in (a–d) correspond to 15 μm. The technique described in (a–d) is used to write MPI-KG (Max-Planck Institute of Colloids and Interfaces (German spelling))(e); the scale bar here corresponds to 20 μm. Reproduced with permission from Ref. 60. Copyright 2010, Wiley-VCH Verlag GmbH.
9. Corrosion protection
Applications of polyelectrolyte Layer-by-Layer deposition spurred interest in the field of corrosion protection as a possible means of replacing toxic Cr(VI) compounds.61 One of the main advantages of polyelectrolyte structures is their light, pH and humidity responsiveness, which would enable a self-healing process by changes of environment that existed at the beginning of the corrosion. Such a system would seem to be useful both for steel alloys62 and non-ferrous materials. 61,63-66
The protective layer can consist of pure polyelectrolytes,64 optionally with corrosion protective additives and could be multi-layered with sol-gel63 and/or corrosion inhibitor coatings.62 A more sophisticated method is based on creating micro and nanocapsules with PE coatings. Capsules can be templated (the template is the dissolvable core) on SiO2 particles,61,67 TiO2, nanoparticles,67 nanotube and pure polyelectrolyte nanocapsules.68,69
Other corrosion protection substances, such as benzotriazole63,65,67 and its derivatives69 and monomers for filling scratches for further induced polymerization can be loaded inside these capsules. They are then incorporated in a more complex matrix such as sol-gel,64,66,70 epoxy69 coatings or polyelectrolyte multilayers.68 Combined, these structures demonstrate mechanical strength, corrosion protection and self-healing processes,71 triggered by the initiation of corrosion or laser irradiation. We highlight here the development of light-sensitive capsule-based active materials.67,72-74 The principle of the approach is shown in Fig. 13.
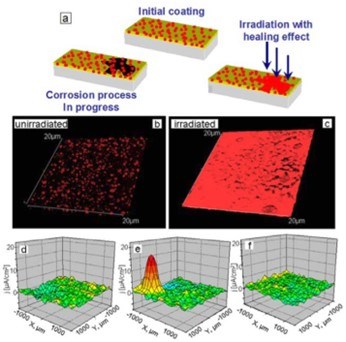
Figure 13 - (a) Schematic of light-responsive protective coating. Luminescent confocal images of polyelectrolyte containers with titania cores incorporated into SiOx-ZrOx films. The images are obtained (b) before and (c) after UV irradiation. The particles in the figures correspond to aggregates of nanocontainers. Three-dimensional (3D) maps of the ionic currents above the surface area correspond to a mechanical defect in sol-gel coating loaded with TiO2(benzotriazole)/(PEI/PSS)2 containers: (d) at the initial moment on the artificial defect; (e) after 64 hr of corrosion; (f) is (e) after UV-irradiation and inhibitor release. Solution: 0.1M NaCl. Reproduced with permission from Ref. 72. Copyright 2009. The Royal Society of Chemistry.
Corrosion mitigating inhibitors encapsulated in the capsule with a mesoporous core and light-controlled permeability of the shell have been used for developing coatings with self-healing functions, which are enabled by controllably releasing the inhibitor upon exposure to laser (infra-red) irradiation. Titania nanoparticles72 can provide multilayers with photo sensitivity. Thus, titania was used both as containers for loading benzotriazole (BTA), for corrosion inhibition and as photosensitive agents. Such capsules were then covered with polyelectrolytes, and they released upon UV light irradiation.
The conformational structure of polyelectrolytes depends on the pH of the environment. Thus, change in pH for certain configurations will result in different values of permeability of the structures. The system can demonstrate both self-healing and self-regulation behavior when the pH changes resulting from the corrosion process starts the self-healing process. The reversible changes of polyelectrolyte permeability can be explained by locally changed solution pH as the consequence of photocatalytic degradation of water on the titania. The end of irradiation causes system relaxation, and the particles return to their initial structure. Visualization of the local pH gradient is possible by modeling physico-chemical properties via a scanning ion-selective electrode technique (SIET).76-78
The inclusion of metal nanoparticles using the LbL procedure enabled the synthesis of materials for other unusual applications. Electroactive (2 nm in diameter) polyelectrolyte-capped Pt nanoparticles assembled into LbL arrays have been used in the catalytic production of H2.78 Photocatalytic reduction79 of noble metal particles on titania cores67 leads to dual-wavelength responsiveness in the UV to near-IR regions of the electromagnetic spectrum. By auto-catalysis, it is possible to regulate photocatalytically waves of enzymatic reactions,80 switch biofilm fluorescence,81 build a platform for a chemical logic device,82 and perform sustainable diagnostics.83-85 The trend in the field of encapsulated systems is to combine different functions in one capsule matrix. One such functionality is to control the release of biocides together with corrosion inhibitors,86 while a potential approach can lead to building self-regulating biocide systems.87
10. Development of sensors and biosensors based on Layer-by-Layer assembled coating
Gold nanoparticles (AuNP) embedded into the polyelectrolyte matrices on optical fiber exhibited a high accuracy pH sensing functionality based on the localized surface plasmon resonance (SPR).88 Measurement of pH shifts89 was also implemented by incorporating gold nanorods which detect the surface plasmon shift of the dispersed nanorods with the pH rise.90 In another application, starch-stabilized silver nanoparticles in 3-n-propylpyridinium silsesquioxane chloride matrices were shown to function as SPR-based electrochemical sensors.91
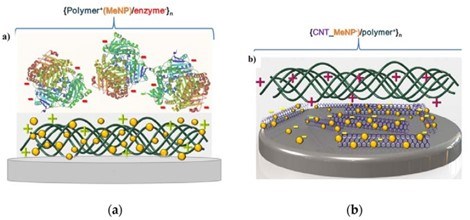
Figure 14 - Schematic diagrams of LbL assemblies of: (a) polymer/nanoparticle (NP)/enzyme and (b) carbon nanotube/NP/polymer sensors. Reproduced with permission from Ref. 94. Copyright 2015 Elsevier.
Hybrid materials assembled using carbon nanotubes and polyelectrolytes were shown to operate as effective membranes for the separation and rejection of ions.92 Furthermore, the high electric conductivity of carbon nanotubes in a polymer framework allowed the development of multi-walled carbon nanotube-based thin film electrodes which were transparent in the mid-IR (infrared) range.93
It can be mentioned that biosensory functions in planar coatings have been developed.94 Figure 14 represents a complementary area to those broadly implemented by polyelectrolyte multilayer capsules.95-97
11. Other nanostructured inorganic building blocks in LbL planar coatings
Recently, a variety of materials were applied to the design of advanced LbL coatings. Quercetin-loaded tripolyphosphate nanoparticles were ordered in a film by an alternative with hyaluronic acid, resulting in a multilayer film capable of improving the anticoagulation properties of surfaces.98 Polysaccharides and nanogels were employed in polyelectrolyte multilayers, showing that the presence of nanogel particles is beneficial for construction of a drug delivery system.99 Linear photochromic norbornene polymers assembled in LbL films exhibited a drastic decrease of the merocyanine band under a prolonged white light irradiation that could potentially be employed as photo-controlled drug delivery system.100 Bioactive thin films were prepared by encapsulation of biomacromolecules such as an enzyme (beta-lactamase) into aluminosilicate halloysite nanotubes and their subsequent use for the fabrication of enzymatic coatings by LbL, that could potentially be used for effluent decontamination.101
The application of quantum dots (QD) in capsules makes it possible to bring the multifunctionality of encapsulation processes and sensor capabilities. The easily adjustable luminescence of QD has a high potential for applying QD-containing polyelectrolyte-based coatings and capsules for biological uses, particularly in medical and materials science as devices and theragnostic agents.102
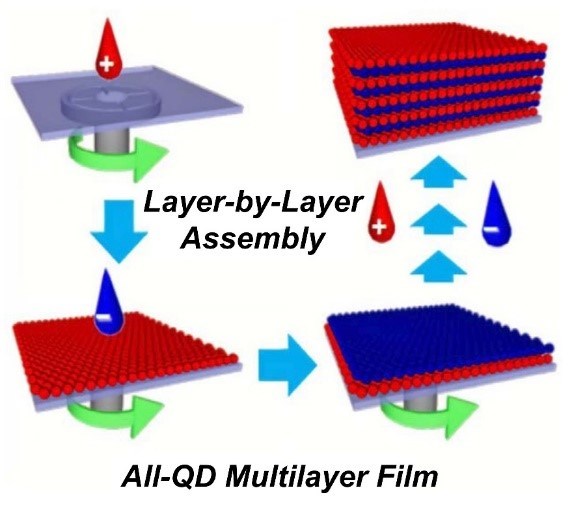
Figure 15 - Schematic diagram for the preparation of all-quantum dot (QD) multilayer films based on spin-assisted Layer-by-Layer assembly, by sequentially depositing oppositely charged QDs (blue QDs and red QDs represent negatively charged QDs (QD-mercaptopropionic acid (MPA)) and positively charged QDs (QD-cysteamine (CAm)), respectively). Reproduced with permission from Ref. 104. Copyright 2010, American Chemical Society.
There are two trends in the design of composite materials based on quantum dots and layer-by-layer techniques. In the first approach, quantum dots are incorporated into Layer-by-Layer films. Here, the QDs are usually chemically modified with thioglycolic acid (TGA),103 mercaptopropionic acid (MPA)104 or mercaptoacetic acid (MAA),105 and they have a negative zeta potential when cysteamine is applied (Fig. 15).This first approach has been conducted by reducing the toxicity of QD, the stabilization of dispersity and the optimization of distribution while the QD optical properties were unaffected.106 CdSe is the typical material for quantum dots used for Layer-by-Layer coating functionalization. For example, MAA-treated QDs have been coated by alternatively applying polyallylamine and polyvinyl sulfonic acid.105
Materials prepared by the second approach find applications in designing flexible organo-electronic devices.107 Stabilization of QDs with polyelectrolytes provides the possibility of the energy transfer between bilayers in charged polymers. The resulting film is reported to be suffciently sensitive for the detection of paraoxon108 or deltamethrin. Incorporation of quantum dots with graphene NPs in an alternative stacking manner in the PAH layers109 leads to augmentation of the separation of charges and transport in a GNs-CdS QDs composite film. A drawback to such methods is that CdSe nanoparticles undergo photooxidation in the polyelectrolyte matrix.
The LbL assembled polyelectrolyte capsules can be functionalized with QD as biocompatible fluorescent agents for live cell targeting.110-112 In this case, the polyelectrolyte film decreases the typical cytotoxic effect of the QD with the fluorescence properties remaining.
A prospective field of engineering structures with semiconductor nanocrystals involves the construction of complex ordered building blocks similar to those used photonic crystals. Thus, demonstrated luminescence in both the IR and visible ranges could be a starting point for development of sophisticated optoelectronics and optical telecommunications devices.
Biologically active QD-based hybrid nanocrystal polyelectrolyte structures with an outer layer of anti-immunoglobulin G(anti-IgG) were shown to render some bio-specific properties to particles113
Considering the interesting info-chemistry,114,115 optical coding and multiplexing can be achieved at different wavelengths and intensities by bringing in QDs in polyelectrolyte multilayers. To achieve that effect, it is possible to create bits of information by tuning the amount of red, green, and blue QD for achieving some characteristic color ration. Combining these structures with some receptors makes it possible to identify various bioprocesses,116 while gradient coatings117 can stimulate combinatorial studies.71,118
LbL assemblies were also functionalized by carbon-based fillers.119 Polyelectrolyte-assisted layer-by -layer fabrication of carbon-containing coatings was proposed to order carbon fillers providing superior film properties. The unique combination of carbon material properties together with versatility of LbL assembly allows the fabrication of multifunctional nanocomposite materials with improved, mechanical, optical, thermal, electromagnetic and electrochemical properties.120 The unique properties of graphene enable high-performance capacitors and effective electromagnetic shielding.121 A wide range of coatings functionalized by carbon fillers is aimed at the development of materials with a gas barrier function.122 The change in permeability of microcapsules tuned by graphene oxide was also applied in drug delivery to reduce the permeability of low molecular substances.123
Furthermore, coatings can be obtained on surfaces with complex shapes to provide additional functionality. For example, coatings containing arrays of closed cavities can be obtained (so-called microchambers) to store and release functional cargo in a controlled manner. Functionalization of microchambers by graphene oxide enable release on demand by a near-infrared (NIR) laser in the therapeutic window (Fig. 16).124
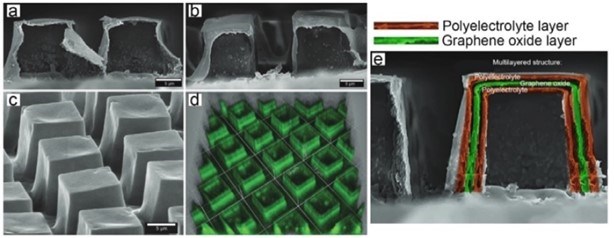
Figure 16 - SEM (scanning electron microscope) images of LbL-assembled microchambers constructed from a pure polyelectrolyte film (a) and functionalized by graphene oxide (b,c) and its corresponding confocal laser scanning microscopy (CLSM) image (d). Reproduced with permission from Ref. 124. Copyright 2018, Wiley-VCH Verlag GmbH. (e) Schematic diagrams of the layered functionalization of microchambers by polyelectrolyte polymers and graphene.
An unusual application of graphene oxide was shown by binary hybrid-filled LbL coatings composed of graphene oxide and β-FeOOH nanorods which enable reduction of flammability of polyurethane foams.125 AuNP assembled in unconventional LbL architectures enabled analysis of hybridization reactions with the ssDNA monitored via methylene blue as the electrochemical indicator. Such an architecture was used in DNA electrochemical biosensors126 along with Pd nanoparticle-based RNA biosensors.127
12. Conclusions and perspectives
At the beginning of research on Layer-by-Layer assembly, nanoparticles carried rather auxiliary functions. In fact, they were simply other additives among available building blocks. The corrosion mitigation/prevention of metallic substrates is not only an engineering effort, but also a fundamental scientific difficulty. Ideally, an anticorrosion coating should be designed to be stimulus-responsive, multifunctional, resistant and durable in a wide range of application.
However, during later research and development phases, nanoparticles emerged as key enabling components, which drove advances and pushed the development of many applications. Nowadays, nanoparticles are broadly used in LbL assembly, which serves as a matrix to order functional components in a predetermined manner with a high special resolution.
This enables a variety of functionalities from smart coatings to drug-delivery systems. Introducing nanoparticles of a different nature into polyelectrolyte matrices is shown to produce alternative materials with superior properties for optical applications (light-emitting materials, light-harvesting materials), sensors and smart coatings, including those with anticorrosion function and improved mechanical properties or barrier function. These advances have enabled the fabrication of multifunctional drug carriers with controllable and switchable parameters for advanced coatings in response to external stimuli.
For hybrid coatings containing nanoparticles, external stimuli have also been shown to control the surface cross-linking, its mechanical properties, corrosion functionality, etc.
Some disadvantages of adding nanoparticles have also been identified. First, adding nanoparticles increases the costs and may prolong the time required for the LbL assembly. In addition, and specifically for biological applications, potential biocompatibility issues need to be addressed for some specific types of nanoparticles, for example for semiconductor quantum dots. That is why the advantages of adding nanoparticles and nanocomponents completely outweigh these potential disadvantages.
In assessing future trends of research and development activities, one can say that nanoparticles have become indispensable components of the LbL assembly. Research work is underway to developed truly advanced coatings both at spherical and flat interfaces. In this regard, LbL-assembly has proved to be a very versatile method for the assembly of coatings with an enormous potential for control of their properties and a rich mix of stimuli available for achieving that.
13. References
1. G. Decher and J.B. Schlenoff, Editors, Multilayer Thin Films: Sequential Assembly of Nanocomposite Materials, Wiley-VCH Verlag, Berlin, 2012.
2. G. Decher and J. Schmitt, “Fine-tuning of the film thickness of ultrathin multilayer film composed of consecutively alternating layers of anionic and cationic polyelectrolytes,” Prog. Colloid Polym. Sci., 89, 160-164 (1992).
3. G. Decher, “Fuzzy nanoassemblies: Toward layered polymeric multicomposites,” Science, 277 (5330), 1232-1237 (1997).
4. E. Donath, G.B. Sukhorukov, F. Caruso, S.A. Davis and H. Möhwald, “Novel hollow polymer shells by colloid-templated assembly of polyelectrolytes,” Angew. Chem. Int. Ed., 37 (16), 2201–2205 (1998).
5. F. Cuomo, F. Lopez, M.G. Miguel and B. Lindman, “Vesicle-templated Layer-by-Layer assembly for the production of nanocapsules,” Langmuir, 26 (13), 10555–10560 (2010).
6. E. Guzmán, H. Ritacco, F. Ortega, T. Svitova, C.J. Radke and R.G. Rubio, “Adsorption kinetics and mechanical properties of ultrathin polyelectrolyte multilayers: liquid-supported versus solid-supported films,” J. Phys. Chem. B, 113 (20), 7128-7137 (2009).
7. G. Rydzek, J-S. Thomann, N. Ben Ameur, L. Jierry, P. Mesini, A. Ponche, C. Contal, A.E. El Haitami, J.C. Voegel, B. Senger, P. Schaaf, B. Frisch and F. Boulmedais, “Polymer multilayer films obtained by electrochemically catalyzed click chemistry,” Langmuir, 26 (4), 2816-2824 (2009).
8. E. Guzmán, R. Chuliá-Jordán, F. Ortega and R.G. Rubio, “Influence of the percentage of acetylation on the assembly of LbL multilayers of poly (acrylic acid) and chitosan,” Phys. Chem. Chem. Phys., 13 (40), 18200-18207 (2011).
9. E. Kharlampieva, V. Kozlovskaya, J.F. Ankner and S.A. Sukhishvili, “Hydrogen-bonded polymer multilayers probed by neutron reflectivity,” Langmuir, 24 (20), 11346–11349 (2008).
10. B. Kumar, Y. T. Park, M. Castro, J.C. Grunlan and J.F. Feller, “Fine control of carbon nanotubes–polyelectrolyte sensors sensitivity by electrostatic Layer-by-Layer assembly (eLbL) for the detection of volatile organic compounds (VOC),” Talanta, 88, 396-402 (2012).
11. E. Guzmán, V. San Miguel, C. Peinado, F. Ortega, and R.G. Rubio, “Polyelectrolyte multilayers containing triblock copolymers of different charge ratio,” Langmuir, 26 (13),11494-11502 (2010).
12. E. Guzmán, J.A. Cavallo, R. Chuliá-Jordán, C. Gómez, M.C. Strumia, F. Ortega and R.G. Rubio, “pH induced changes in the fabrication of multilayers of poly (acrylic acid) and chitosan: fabrication, properties, and tests as a drug storage and delivery system, “Langmuir, 27 (11), 6836-6845 (2011).
13. L.L. del Mercato, M.M. Ferraro, F. Baldassarre, S. Mancarella, V. Greco, R. Rinaldi R, and S. Leporatti, “Biological applications of LbL multilayer capsules: from drug delivery to sensing,” Adv. Colloid Interface Sci., 207, 139-154 (2014).
14. M. Keeney, X.Y. Jiang, M. Yamane, M. Lee, S. Goodman and F. Yang, “Nanocoating for biomolecule delivery using Layer-by-Layer self-assembly,” J. Mat. Chem. B, 3 (45), 8757-8770 (2015).
15. N. Aggarwal, N. Altgärde, S. Svedhem, K. Zhang, S. Fischer and T. Groth, “Study on multilayer structures prepared from heparin and semi-synthetic cellulose sulfates as polyanions and their influence on cellular response,” Colloids and Surf. B: Biointerfaces, 116, 93-103 (2014).
16. B.B. Hsu, S.R. Hagerman and P.T. Hammond, “Rapid and efficient sprayed multilayer films for controlled drug delivery,” J. Appl. Polym. Sci., 133 (25), 43563 (2016).
17. A. Zhuk, R. Mirza and S. Sukhishvili, “Multiresponsive clay-containing Layer-by-Layer films,” ACS Nano, 5 (11), 8790-8799 (2011).
18. A.P.R. Johnston, C. Cortez-Jugo, A.S. Angelatos and F. Caruso, “Layer-by-layer engineered capsules and their applications,” Curr. Opin. Colloid & Interface Sci., 11 (4), 203-209 (2006).
19. W. Tong, X. Song and C. Gao, “Layer-by-layer assembly of microcapsules and their biomedical applications,” Chem. Soc. Rev., 41 (18), 6103-6124 (2012).
20. J.M. Silva, R.L. Reis and J.F. Mano, “Biomimetic extracellular environment based on natural origin polyelectrolyte multilayers,” Small, 12 (32), 4308-4342 (2016).
21. F-X. Xiao, M. Pagliaro, Y-J. Xu and B. Liu, “Layer-by-layer assembly of versatile nanoarchitectures with diverse dimensionality: a new perspective for rational construction of multilayer assemblies,” Chem. Soc. Rev., 45 (11), 3088-3121 (2016).
22. R. Von Klitzing, “Internal structure of polyelectrolyte multilayer assemblies,” Phys. Chem. Chem. Phys., 8 (43), 5012-5033 (2006).
23. E. Guzmán, H.A. Ritacco, F. Ortega and R.G. Rubio, “Growth of polyelectrolyte layers formed by poly (4-styrenesulfonate sodium salt) and two different polycations: new insights from study of adsorption kinetics,” J. Phys. Chem. C, 116 (29), 15474-15483 (2012).
24. E. Guzmán, H. Ritacco, J.E.F. Rubio, R.G. Rubio and F. Ortega, “Salt-induced changes in the growth of polyelectrolyte layers of poly(diallyldimethylammoniumchloride) and poly (4-styrene sulfonate of sodium),” Soft Matter, 5 (10), 2130-2142 (2009).
25. E. Guzmán, A. Maestro, S. Llamas, J. Álvarez-Rodríguez, F. Ortega, Á. Maroto-Valiente, and R.G. Rubio, “3D solid supported inter-polyelectrolyte complexes obtained by the alternate deposition of poly (diallyldimethylammonium chloride) and poly (sodium 4-styrenesulfonate),” Beilstein J. Nanotech., 7 , 197-208 (2016).
26. J.B. Schlenoff and S.T. Dubas, “Mechanism of polyelectrolyte multilayer growth: charge overcompensation and distribution,” Macromolecules, 34 (3), 592-598 (2001).
27. M. Salomäki, P. Tervasmäki, S. Areva and J. Kankare, “The Hofmeister anion effect and the growth of polyelectrolyte multilayers,” Langmuir, 20 (9), 3679-3683 (2004).
28. M. Salomäki, I.A. Vinokurov and J. Kankare, “Effect of temperature on the buildup of polyelectrolyte multilayers,” Langmuir, 21 (24), 11232-11240 (2005).
29. Y. Xiang, S. Lu and S.P. Jiang, “Layer-by-layer self-assembly in the development of electrochemical energy conversion and storage devices from fuel cells to supercapacitors,” Chem. Soc. Rev., 41 (21), 7291-7321 (2012).
30. Y. Li, X. Wang and J. Sun, “Layer-by-layer assembly for rapid fabrication of thick polymeric films,” Chem. Soc. Rev., 41 (18), 5998-6009 (2012).
31. G.B. Sukhorukov, E. Donath, H. Lichtenfeld, E. Knippel, M. Knippel, A. Budde and H. Möhwald, “Layer-by-layer self-assembly of polyelectrolytes on colloidal particles,” Colloids Surf. A, 137 (1-3), 253-266 (1998).
32. H.G. Bagaria and W.S. Wong, “Polyamine-salt aggregate assembly of capsules as responsive drug delivery vehicles,” J. Mater. Chem., 21 (26), 9454-9466 (2011).
33. U. Bazylińska, P. Warszyński and K.A. Wilk, ”Influence of pH upon in vitro sustained dye release from oil-core nanocapsules with multilayer shells,” Colloids Surf. A: Physicochemical and Engineering Aspects, 413, 266-272 (2012).
34. E. Guzmán, M. Ruano, F. Ortega and R.G. Rubio, “Stratified interpolyelectrolyte complexes: Fabrication, structure and properties,” In: P.M. Visakh, O. Bayraktar and G.A. Picó, Editors, Polyelectrolytes, Springer International Publishing, Cham, Switzerland, 2014; pp. 299–347.
35. K. Szczepanowicz, U. Bazylińska, J. Pietkiewicz, L. Szyk-Warszyńska, K.A. Wilk and P. Warszyński, “Biocompatible long-sustained release oil-core polyelectrolyte nanocarriers: from controlling physical state and stability to biological impact,” Adv. Colloid Interface Sci., 222, 678-691 (2015).
36. S.T. Dubas and J.B. Schlenoff, “Factors controlling the growth of polyelectrolyte multilayers,” Macromolecules, 32 (24), 8153-8160 (1999).
37. U. Voigt, V. Khrenov, K. Tauer, M. Hahn, W. Jaeger and R. von Klitzing, “The effect of polymer charge density and charge distribution on the formation of multilayers,” J. Phys.: Condens. Matter, 15 (1), S213-S218 (2003).
38. E. Poptoshev, B. Schoeler and F. Caruso, “Influence of solvent quality on the growth of polyelectrolyte multilayers,” Langmuir, 20 (3), 829-834 (2004).
39. H.H. Rmaile and J.B. Schlenoff, “Internal pKa's in polyelectrolyte multilayers: coupling protons and salt,” Langmuir, 18 (22), 8263-8265 (2002).
40. N.A. Kotov, “Layer-by-layer self-assembly: the contribution of hydrophobic interactions,” Nanostr. Mat., 12 (5-8), 789-796 (1999).
41. G. Ladam, P. Schaad, J.C. Voegel, P. Schaaf, G. Decher and F. Cuisinier, “In situ determination of the structural properties of initially deposited polyelectrolyte multilayers,” Langmuir, 16 (3), 1249-1255 (1999).
42. N. Cini, T. Tulun, G. Decher and V. Ball, “Step-by-step assembly of self-patterning polyelectrolyte films violating (almost) all rules of layer-by-layer deposition,” J. Am. Chem. Soc., 132 (24), 8264-8265 (2010).
43. C. Picart, P. Lavalle, P. Hubert, F.J.G. Cuisinier, G. Decher, P. Schaaf and J.-C. Voegel, “Buildup mechanism for poly(L-lysine)/hyaluronic acid films onto a solid surface,” Langmuir, 17 (23), 7414-7424 (2001).
44. P. Lavalle, C. Gergely, F.J.G. Cuisinier, G. Decher, P. Schaaf, J.-C. Voegel and C. Picart, “Comparison of the structure of polyelectrolyte multilayer films exhibiting a linear and an exponential growth regime: an in situ atomic force microscopy study,” Macromolecules, 35 (11), 4458-4465 (2002).
45. A. Schneider, L. Richert, G. Francius, J.-C. Voegel, and C. Picart, “Elasticity, biodegradability and cell adhesive properties of chitosan/hyaluronan multilayer films,” Biomed. Mater., 2 , S45-S51 (2007).
46. C. Picart, J. Mutterer, L. Richert, Y. Luo, G.D. Prestwich, P. Schaaf, J.-C. Voegel and P. Lavalle, “Molecular basis for the explanation of the exponential growth of polyelectrolyte multilayers,” Proc. Nat. Acad. Sci. USA, 99 (20), 12531-12535 (2002).
47. P. Lavalle, C. Picart, J. Mutterer, C. Gergely, H. Reiss, J.-C. Voegel, B. Senger and P. Schaaf, “Modeling the buildup of polyelectrolyte multilayer films having exponential growth,” J. Phys. Chem. B, 108 , 635-648 (2003).
48. R.A. McAloney, M. Sinyor, V. Dudnik and M.C. Goh, “Atomic force microscopy studies of salt effects on polyelectrolyte multilayer film morphology,” Langmuir, 17 (21),6655-6663 (2001).
49. S. Srivastava and N.A. Kotov, “Composite Layer-by-Layer (LbL) assembly with inorganic nanoparticles and nanowires,” Acc. Chem. Res., 41 , 1831-1841 (2008).
50. Y. Zhou, M. Cheng, X. Zhu, Y. Zhang, Q. An, and F. Shi, “A facile method for the construction of stable polymer–inorganic nanoparticle composite multilayers,” J. Mater. Chem. A, 1 (37), 11329-11334 (2013).
51. M.M. Abd El-Hady, S. Sharaf and A. Farouk, “Highly hydrophobic and UV protective properties of cotton fabric using Layer-by-Layer self-assembly technique,” Cellulose, 27 (2), 1099-1110 (2020).
52. N.J. Abuid, K.M. Gattás-Asfura, E.A. Schofield and C.L. Stabler, “Layer-by-Layer Cerium Oxide Nanoparticle Coating for Antioxidant Protection of Encapsulated Beta Cells,” Adv. Healthcare Mater., 8 (12), e1801493 (2019).
53. R.A. Ghostine, R.M. Jisr, A. Lehaf and J.B. Schlenoff, “Roughness and Salt Annealing in a Polyelectrolyte Multilayer,” Langmuir, 29 (37), 11742-11750 (2013).
54. C.C. Buron, C. Filiâtre, F. Membrey, C. Bainier, L. Buisson, D. Charraut and A. Foissy, “Surface morphology and thickness of a multilayer film composed of strong and weak polyelectrolytes: Effect of the number of adsorbed layers, concentration and type of salts,” Thin Solid Films, 517 (8), 2611-2617 (2009).
55. P. Lavalle, C. Gergely, F.J.G. Cuisinier, G. Decher, P. Schaaf, J-C. Voegel and C. Picart, “Comparison of the Structure of Polyelectrolyte Multilayer Films Exhibiting a Linear and an Exponential Growth Regime: An in Situ Atomic Force Microscopy Study,” Macromolecules, 35 (11), 4458-4465 (2002).
56. A. Mzyk, J.M. Lackner, P. Wilczek, L. Lipinska, A. Niemiec-Cyganek, A. Samotus and M. Morenc, “Polyelectrolyte multilayer film modification for chemo-mechano-regulation of endothelial cell response,” RSC Adv., 6 (11), 8811-8828 (2016).
57. A. Schneider, G. Francius, R. Obeid, P. Schwinté, J. Hemmerlé, B. Frisch, P. Schaaf, J-C. Voegel, B. Senger and C. Picart, “Polyelectrolyte Multilayers with a Tunable Young’s Modulus: Influence of Film Stiffness on Cell Adhesion,” Langmuir, 22 (3), 1193-1200 (2006).
58. S. Schmidt, N. Madaboosi, K. Uhlig, D. Köhler, A. Skirtach, C. Duschl, H. Möhwald and D.V. Volodkin, “Control of cell adhesion by mechanical reinforcement of soft polyelectrolyte films with nanoparticles,” Langmuir, 28 (18), 7249-7257 (2012).
59. A.A. Abalymov, B.V. Parakhonskiy and A.G. Skirtach, “Colloids-at-surfaces: Physicochemical approaches for facilitating cell adhesion on hybrid hydrogels,” Colloids Surf. A Physicochem. Eng. Aspects, 603, e125185 (2020).
60. A.G. Skirtach, D.V. Volodkin, and M.H. Möhwald, “Bio-interfaces-Interaction of PLL/HA Thick Films with Nanoparticles and Microcapsules,” ChemPhysChem, 11 (4), 822-829 (2010).
61. D.G. Shchukin, M.L. Zheludkevich, K. Yasakau, S. Lamaka, M.G.S. Ferreira and H. Möhwald, “Layer-by-Layer Assembled Nanocontainers for Self-Healing Corrosion Protection,” Adv. Mater., 18 (13), 1672-1678 (2006).
62. D.V. Andreeva, E.V. Skorb and D.G. Shchukin, “Layer-by-Layer Polyelectrolyte/Inhibitor Nanostructures for Metal Corrosion Protection,” ACS Appl. Mater. Interfaces, 2 (7), 1954-1962 (2010).
63. D.V. Andreeva, D. Fix, H. Möhwald and D.G. Shchukin, “Self-Healing Anticorrosion Coatings Based on pH-Sensitive Polyelectrolyte/Inhibitor Sandwichlike Nanostructures,” Adv. Mater., 20 (14), 2789-2794 (2008).
64. D.V. Andreeva, D. Fix, H. Möhwald and D.G. Shchukin, “Buffering polyelectrolyte multilayers for active corrosion protection,” J. Mater. Chem., 18 (15), 1738-1740 (2008).
65. S. Haneder, E. Da Como, J. Feldmann, J.M. Lupton, C. Lennartz, P. Erk, E. Fuchs, O. Molt, I. Münster, C. Schildknecht, C. and G. Wagenblast, “Controlling the Radiative Rate of Deep-Blue Electrophosphorescent Organometallic Complexes by Singlet-Triplet Gap Engineering,” Adv. Mater., 20 (17), 3325-3330 (2008).
66. D.O. Grigoriev, K. Köhler, E. Skorb, D.G. Shchukin and H. Möhwald, “Polyelectrolyte complexes as a “smart” depot for self-healing anticorrosion coatings,” Soft Matter, 5 (7), 1426-1432 (2009).
67. E.V. Skorb, A.G. Skirtach, D.V. Sviridov, D.G. Shchukin and H. Möhwald, “Laser-Controllable Coatings for Corrosion Protection, ACS Nano., 3 (7), 1753-1760 (2009).
68. A.H. Jafari, S.M.A. Hosseini and E. Jamalizadeh, “Investigation of Smart Nanocapsules Containing Inhibitors for Corrosion Protection of Copper,” Electrochim. Acta, 55 (28), 9004-9009 (2010).
69. M. Kopeć, K. Szczepanowicz, G. Mordarski, K. Podgórna, R.P. Socha, P. Nowak, P. Warszyński and T. Hack, “Self-healing epoxy coatings loaded with inhibitor-containing polyelectrolyte nanocapsules,” Prog. Org. Coat., 84, 97-106 (2015).
70. X. Zhao, S. Yuan, Z. Jin, B. Zhang, N. Liu, S. Chen, S. Liu, X. Sun and J. Duan, “Perfect Combination of LbL with Sol-Gel Film to Enhance the Anticorrosion Performance on Al Alloy under Simulated and Accelerated Corrosive Environment,” Materials, 13 (1), 111 (2019).
71. F. Fan, C. Zhou, X. Wang and J. Szpunar, “Layer-by-Layer Assembly of a Self-Healing Anticorrosion Coating on Magnesium Alloys,” ACS Appl. Mater. Interfaces, 7 (49), 27271-27278 (2015).
72. E.V. Skorb, D.V. Sviridov, H. Möhwald and D.G. Shchukin, “Light responsive protective coatings,” Chem. Commun. 2009 (40), 6041-6043 (2009).
73. E.V. Skorb, D. Fix, D.V. Andreeva, H. Möhwald and D.G. Shchukin, “Surface-Modified Mesoporous SiO2 Containers for Corrosion Protection,” Adv. Funct. Mater., 19 (15), 2373-2379 (2009).
74. E.V. Skorb, D.G. Shchukin, H. Möhwald and D.V. Sviridov, “Photocatalytically-active and photocontrollable coatings based on titania-loaded hybrid sol–gel films,” J. Mater. Chem., 19, 4931-4937 (2009).
75. S.A. Ulasevich, G. Brezesinski, H. Möhwald, P. Fratzl, F.H. Schacher, S.K. Poznyak, D.V. Andreeva and E.V. Skorb, “Light-Induced Water Splitting Causes High-Amplitude Oscillation of pH-Sensitive Layer-by-Layer Assemblies on TiO2,” Angew. Chem. Int. Ed., 55 , 13001-13004 (2016).
76. H.M. Maltanava, S.K. Poznyak, D.V. Andreeva, M.C. Quevedo, A.C. Bastos, J. Tedim, M.G.S. Ferreira and E.V. Skorb, “Light-Induced Proton Pumping with a Semiconductor: Vision for Photoproton Lateral Separation and Robust Manipulation,” ACS Appl. Mater. Interfaces, 9 (28), 24282-24289 (2017).
77. N.V. Ryzhkov and E.V. Skorb, “A platform for light-controlled formation of free-stranding lipid membranes,” J. R. Soc. Interface, 17 (163), 20190740 (2020).
78. G.E. Fenoy, E. Maza, E. Zelaya, W.A. Marmisollé and O. Azzaroni, “Layer-by-layer assemblies of highly connected polyelectrolyte capped-Pt nanoparticles for electrocatalysis of hydrogen evolution reaction,” Appl. Surf. Sci., 416, 24-32 (2017).
79. E.V. Skorb, L.I. Antonouskaya, N.A. Belyasova, D.G. Shchukin, H. Möhwald and D.V. Sviridov, “Antibacterial activity of thin-film photocatalysts based on metal-modified TiO2 and TiO2:In2O3 nanocomposite,” Appl. Catal. B Environ., 84 (1-2), 94-99 (2008).
80. Y. Lanchuk, A. Nikitina, N. Brezhneva, S.A. Ulasevich, S.N. Semenov and E.V. Skorb, “Photocatalytic Regulation of an Autocatalytic Wave of Spatially Propagating Enzymatic Reactions,” ChemCatChem, 10 (8), 1798-1803 (2018).
81. A.A. Nikitina, S.A. Ulasevich, I.S. Kassirov, E.A. Bryushkova, E.I. Koshel and E.V. Skorb, “Nanostructured Layer-by-Layer Polyelectrolyte Containers to Switch Biofilm Fluorescence,” Bioconjugate. Chem., 29 (11), 3793-3799.
82. N.V. Ryzhkov, V.Y. Yurova, S.A. Ulasevich and E.V. Skorb, “Photoelectrochemical photocurrent switching effect on a pristine anodized Ti/TiO2 system as a platform for chemical logic devices,” RSC Adv., 10 (21), 12355-12359 (2020).
83. Y.V. Lanchuk, S.A. Ulasevich, T.A. Fedotova, D.M. Kolpashchikov and E.V. Skorb, “Towards sustainable diagnostics: Replacing unstable H2O2 by photoactive TiO2 in testing systems for visible and tangible diagnostics for use by blind people,” RSC Adv., 8 (66), 37735-37739 (2018).
84. A.A. Stekolshchikova, A.V. Radaev, O.Y. Orlova, K.G. Nikolaev and E.V. Skorb, “Thin and Flexible Ion Sensors Based on Polyelectrolyte Multilayers Assembled onto the Carbon Adhesive Tape,” ACS Omega, 4 (13), 15421-15427 (2019).
85. K.G. Nikolaev, E.V. Kalmykov, D.O. Shavronskaya, A.A. Nikitina, A.A. Stekolshchikova, E.A. Kosareva, A.A. Zenkin, I.S. Pantiukhin, O.Y. Orlova, A.V. Skalny and E.V. Skorb, “ElectroSens Platform with a Polyelectrolyte-Based Carbon Fiber Sensor for Point-of-Care Analysis of Zn in Blood and Urine,” ACS Omega, 5 (30), 18987-18994 (2020).
86. D.V. Andreeva, D.V Sviridov, A. Masic, H. Möhwald and E.V. Skorb, “Nanoengineered Metal Surface Capsules: Construction of a Metal-Protection System, Small, 8 (6), 820-825 (2012).
87. J. Gensel, T. Borke, N.P. Pérez, A. Fery, D.V. Andreeva, E. Betthausen, A.H.E. Müller, H. Möhwald and E.V. Skorb, “Cavitation Engineered 3D Sponge Networks and Their Application in Active Surface Construction,” Adv. Mater., 24 (7), 985-989 (2012).
88. P.J. Rivero, J. Goicoechea, M. Hernaez, A.B. Socorro, I.R. Matias and F.J. Arregui, “Optical fiber resonance-based pH sensors using gold nanoparticles into polymeric Layer-by-Layer coatings,” Microsyst. Technol., 22 (7), 1821-1829 (2016).
89. I. Tokarev, I. Tokareva and S. Minko, “Optical Nanosensor Platform Operating in Near-Physiological pH Range via Polymer-Brush-Mediated Plasmon Coupling,” ACS Appl. Mater. Interfaces, 3 , 143-146 (2011).
90. V. Kozlovskaya, E. Kharlampieva, B.P. Khanal, P. Manna, E.R. Zubarev and V.V. Tsukruk, “Ultrathin Layer-by-Layer Hydrogels with Incorporated Gold Nanorods as pH-Sensitive Optical Materials,” Chem. Mater., 20 (24), 7474-7485 (2008).
91. R.D. De Oliveira, G.N. Calaça, C.S. Santos, S.T. Fujiwara and C.A. Pessôa, “Preparation, characterization and electrochemistry of Layer-by-Layer films of silver nanoparticles and silsesquioxane polymer,” Colloids Surf. A Physicochem. Eng. Asp., 509, 638-647 (2016).
92. J. Irigoyen, T. Laakso, N. Politakos, L. Dahne, A. Pihlajamäki, M. Mänttäri and S.E. Moya, “Design and Performance Evaluation of Hybrid Nanofiltration Membranes Based on Multiwalled Carbon Nanotubes and Polyelectrolyte Multilayers for Larger Ion Rejection and Separation,” Macromol. Chem. & Phys., 217 (6), 804-811 (2016).
93. J. Stanojev, B. Bajac, Z. Cvejić, J. Matović and V.V. Srdić, “Development of MWCNT thin film electrode transparent in the mid-IR range,” Ceram. Int., 46 (8A), 11340-11345 (2020).
94. M.M. Barsan and C.M.A. Brett, “Recent advances in Layer-by-Layer strategies for biosensors incorporating metal nanoparticles,” TrAC Trends Anal. Chem., 79, 286-296 (2016).
95. M. McShane and D. Ritter, “Microcapsules as optical biosensors,” J. Mater. Chem., 20 , 8189-8193 (2010).
96. G.B. Sukhorukov, A.L. Rogach, M. Garstka, S. Springer, W.J. Parak, A. Muñoz-Javier, O. Kreft, A.G. Skirtach, A.S. Susha, Y. Ramaye, R. Palankar and M. Winterhalter, ”Multifunctionalized polymer microcapsules: Novel tools for biological and pharmacological applications,” Small, 3 (6), 944-955 (2007).
97. L. Van der Meeren, J. Li, B.V. Parakhonskiy, D.V. Krysko and A.G. Skirtach, “Classification of analytics, sensorics, and bioanalytics with polyelectrolyte multilayer capsules,” Anal. Bioanal. Chem., 412, 5015-5029 (2020).
98. X. Wu, C. Liu, H. Chen, Y. Zhang, L. Li and N. Tang, “Layer-by-Layer Deposition of Hyaluronan and Quercetin-Loaded Chitosan Nanoparticles onto Titanium for Improving Blood Compatibility,” Coatings, 10 (3), 256 (2020).
99. S. Sydow, D. de Cassan, R. Hänsch, T.R. Gengenbach, C.D. Easton, H. Thissen and H. Menzel, “Layer-by-layer deposition of chitosan nanoparticles as drug-release coatings for PCL nanofibers,” Biomater. Sci., 7 (1), 233-246 (2019).
100. P.P. Campos, A. Dunne, C. Delaney, C. Moloney, S.E. Moulton, F. Benito-Lopez, M. Ferreira, D. Diamond and L. Florea, “Photoswitchable Layer-by-Layer Coatings Based on Photochromic Polynorbornenes BearinSpiropyran Side Groups,” Langmuir, 34 , 4210-4216 (2018).
101. P. Rouster, M. Dondelinger, M. Galleni, B. Nysten, A.M. Jonas and K. Glinel, “Layer-by-layer assembly of enzyme-loaded halloysite nanotubes for the fabrication of highly active coatings,” Colloids Surf. B: Biointerfaces, 178 , 508-514 (2019).
102. A.L. Rogach (Ed.), Semiconductor Nanocrystal Quantum Dots, Springer Vienna: Vienna, Austria, 2008; ISBN 978-3-211-75235-7.
103. S. Abd Rahman, N. Ariffin, N. Azah Yusof, J. Abdullah, F. Mohammad, Z. Ahmad Zubir and N.M.A. Nik Abd Aziz, “Thiolate-Capped CdSe/ZnS Core-Shell Quantum Dots for the Sensitive Detection of Glucose,” Sensors, 17 (7), 1537 (2017).
104. W.K. Bae, J. Kwak, J. Lim, D. Lee, M.K. Nam, K. Char, C. Lee and S. Lee, “Multicolored Light-Emitting Diodes Based on All-Quantum-Dot Multilayer Films Using Layer-by-Layer Assembly Method,” Nano Lett., 10 (7), 2368-2373 (2010).
105. S. Jaffar, K.T. Nam, A. Khademhosseini, J. Xing, R.S. Langer and A.M. Belcher, “Layer-by-Layer Surface Modification and Patterned Electrostatic Deposition of Quantum Dots,” Nano Lett., 4 (8), 1421-1425 (2004).
106. A.T. Nagaraja, A. Sooresh, K.E. Meissner and M.J. McShane, “Processing and Characterization of Stable, pH-Sensitive Layer-by-Layer Modified Colloidal Quantum Dots,” ACS Nano, 7 (7), 6194-6202 (2013).
107. D. Zimnitsky, C. Jiang, J. Xu, Z. Lin, L. Zhang and V.V. Tsukruk, “Photoluminescence of a Freely Suspended Monolayer of Quantum Dots Encapsulated into Layer-by-Layer Films,” Langmuir, 23 (20), 10176-10183 (2007).
108. C.A. Constantine, K.M. Gattás-Asfura, S.V. Mello, G. Crespo, V. Rastogi, T-C. Cheng, J.J. DeFrank and R.M. Leblanc, “Layer-by-Layer Biosensor Assembly Incorporating Functionalized Quantum Dots,” Langmuir, 19 (23), 9863-9867 (2003).
109. F-X. Xiao, J. Miao and B. Liu, “Layer-by-Layer Self-Assembly of CdS Quantum Dots/Graphene Nanosheets Hybrid Films for Photoelectrochemical and Photocatalytic Applications,” J. Am. Chem. Soc., 136 (4), 1559-1569 (2014).
110. G. Nifontova, F. Ramos-Gomes, M. Baryshnikova, F. Alves, I. Nabiev and A. Sukhanova, “Cancer Cell Targeting With Functionalized Quantum Dot-Encoded Polyelectrolyte Microcapsules,” Front. Chem., 7, 34 (2019).
111. G. Nifontova, A, Efimov, O. Agapova, I. Agapov, I. Nabiev and A. Sukhanova, “Bioimaging Tools Based on Polyelectrolyte Microcapsules Encoded with Fluorescent Semiconductor Nanoparticles: Design and Characterization of the Fluorescent Properties,” Nanoscale Res. Lett., 14, 29 (2019).
112. M. Adamczak, H.J. Hoel, G. Gaudernack, J. Barbasz, K. Szczepanowicz and P. Warszyński, “Polyelectrolyte multilayer capsules with quantum dots for biomedical applications,” Colloids Surf. B: Biointerfaces, 90, 211-216 (2012).
113. D. Wang, A.L. Rogach and F. Caruso, “Semiconductor Quantum Dot-Labeled Microsphere Bioconjugates Prepared by Stepwise Self-Assembly,” Nano Lett., 2 (8), 857-861 (2002).
114. M. Hashimoto, J. Feng, R.L. York, A.K. Ellerbee, G. Morrison, S.W. Thomas III, L. Mahadevan and G.M. Whitesides, “Infochemistry: Encoding Information as Optical Pulses Using Droplets in a Microfluidic Device,” J. Am. Chem. Soc., 131 (34), 12420-12429 (2009).
115. N.V. Ryzhkov, D.V. Andreeva and E.V. Skorb, “Coupling pH-Regulated Multilayers with Inorganic Surfaces for Bionic Devices and Infochemistry,” Langmuir, 35 (26), 8543-8556 (2019).
116. M. Han, X. Gao, J.Z. Su and S. Nie, “Quantum-dot-tagged microbeads for multiplexed optical coding of biomolecules,” Nat. Biotechnol., 19, 631-635 (2001).
117. B-E. Pinchasik, K. Tauer, H. Möhwald and A.G. Skirtach, “Polymer Brush Gradients by Adjusting the Functional Density Through Temperature Gradient,” Adv. Mater. Interfaces, 1 (2), 1300056 (2014).
118. M. Sailer and C.J. Barrett, “Fabrication of Two-Dimensional Gradient Layer-by-Layer Films for Combinatorial Biosurface Studies,” Macromolecules, 45 (14), 5704-5711 (2012).
119. K. Ariga, E. Ahn, M. Park and B. Kim, “Layer-by-Layer Assembly: Recent Progress from Layered Assemblies to Layered Nanoarchitectonics,” Chem. An Asian J., 14 (15), 2553-2566 (2019).
120. A. Yu, X. Zhang, H. Zhang, D. Han and A.R. Knight, “Preparation and electrochemical properties of gold nanoparticles containing carbon nanotubes-polyelectrolyte multilayer thin films,” Electrochim. Acta, 56 (25), 9015-9019.
121. C. Vallés, X. Zhang, J. Cao, F. Lin, R.J. Young, A. Lombardo, A.C. Ferrari, L. Burk, R. Mülhaupt and I.A. Kinloch, “Graphene/Polyelectrolyte Layer-by-Layer Coatings for Electromagnetic Interference Shielding,” ACS Appl. Nano Mater., 2 (8), 5272-5281 (2019).
122. H. Liu, P. Bandyopadhyay, T. Kshetri, N.H. Kim, B-C. Ku, B. Moon and J.H. Lee, “Layer-by-layer assembled polyelectrolyte-decorated graphene multilayer film for hydrogen gas barrier application,” Compos. Part B: Eng., 114, 339-347 (2017).
123. R. Kurapati and A.M. Raichur, “Graphene oxide based multilayer capsules with unique permeability properties: Facile encapsulation of multiple drugs,” Chem. Commun., 48, 6013-6015 (2012).
124. A. Ermakov, S.H. Lim, S. Gorelik, A.P. Kauling, R.V.B. de Oliveira, A.H. Castro Neto, E. Glukhovskoy, D.A. Gorin, G.B. Sukhorukov and M.V. Kiryukhin, “Polyelectrolyte-Graphene Oxide Multilayer Composites for Array of Microchambers which are Mechanically Robust and Responsive to NIR Light,” Macromol. Rapid Commun., 40 (5), 1700868 (2019).
125. H. Pan, Y. Lu, L. Song, X. Zhang and Y. Hu, “Construction of Layer-by-Layer coating based on graphene oxide/β-FeOOH nanorods and its synergistic effect on improving flame retardancy of flexible polyurethane foam,” Compos. Sci. Technol., 129, 116-122 (2016).
126. H. Gao, X. Qi, Y. Chen and W. Sun, “Electrochemical deoxyribonucleic acid biosensor based on the self-assembly film with nanogold decorated on ionic liquid modified carbon paste electrode,” Anal. Chim. Acta, 704 (1-2), 133-138 (2011).
127. X. Wu, Y. Chai, R. Yuan, H. Su and J. Han, “A novel label-free electrochemical microRNA biosensor using Pd nanoparticles as enhancer and linker,” Analyst, 138 (4), 1060-1066 (2013).
14. Additional reading
1. J.F. Joanny, “Polyelectrolyte adsorption and charge inversion,” Eur. Phys. J. B, 9, 117-122 (1999).
2. F. Caruso, E. Donath and H. Möhwald, “Influence of polyelectrolyte multilayer coatings on Förster resonance energy transfer between 6-carboxyfluorescein and rhodamine B-labeled particles in aqueous solution, J. Phys. Chem. B, 102 (11), 2011-2016 (1998).
3. S. Schwarz, K.J. Eichhorn, E. Wischerhoff and A. Laschewsky, “Polyelectrolyte adsorption onto planar surfaces: a study by streaming potential and ellipsometry measurements,” Colloids Surf. A, 159 (2-3), 491-501 (1999).
4. A.Y. Grosberg, T.T. Nguyen and B.I. Shklovskii, “Colloquium: the physics of charge inversion in chemical and biological systems,” Rev. Mod. Phys., 74 (2), 329-345 (2002).
5. R.A. Ghostine, M.Z. Markarian and B. Schlenoff, “Asymmetric growth in polyelectrolyte multilayers,” J. Am. Chem. Soc., 135 (20), 7636-7646 (2013).
6. A.M. Lehaf, H.H. Hariri and J.B. Schlenoff, “Homogeneity, modulus, and viscoelasticity of polyelectrolyte multilayers by nanoindentation: refining the buildup mechanism, Langmuir, 28 (15), 6348-6355 (2012).
7. R.A. Ghostine, R.M. Jisr, A. Lehaf and J.B. Schlenoff, “Roughness and salt annealing in a polyelectrolyte multilayer,” Langmuir, 29 (37), 11742-11750 (2013).
8. F.S. Gittleson, D. Hwang, W-H. Ryu, S.M. Hashmi, J. Hwang, T. Goh and A.D. Taylor, “Ultrathin Nanotube/Nanowire Electrodes by Spin–Spray Layer-by-Layer Assembly: A Concept for Transparent Energy Storage, ACS Nano, 9 (10), 10005-10017 (2015).
9. J. Danglad-Flores, K. Eftekhari, A.G. Skirtach and H. Riegler, “Controlled Deposition of Nanosize and Microsize Particles by Spin-Casting, Langmuir, 35 (9), 3404-3412 (2019).
10. H. Hu, M. Pauly, O. Felix and G. Decher, “Spray-assisted alignment of Layer-by-Layer assembled silver nanowires: A general approach for the preparation of highly anisotropic nano-composite films,” Nanoscale, 9 (3), 1307-1314 (2017).
11. J. Campbell and A.S. Vikulina, “Layer-By-Layer Assemblies of Biopolymers: Build-Up, Mechanical Stability and Molecular Dynamics,” Polymers, 12 (9), 1949 (2020).
12. D. Kovačević, R. Pratnekar, K. Godić Torkar, J. Salopek, G. Dražić, A. Abram and K. Bohinc, “Influence of Polyelectrolyte Multilayer Properties on Bacterial Adhesion Capacity,” Polymers, 8 (10), 345 (2016).
13. S. Guo, M.Y. Kwek, Z.Q. Toh, D. Pranantyo, E-T. Kang, X.J. Loh, X. Zhu, D. Jańczewski and K.G. Neoh, “Tailoring Polyelectrolyte Architecture to Promote Cell Growth and Inhibit Bacterial Adhesion,” ACS Appl. Mater. Interfaces, 10 (9), 7882-7891 (2018).
14. A.L. Carvalho, A.C. Vale, M.P. Sousa, A.M. Barbosa, E. Torrado, J.F. Mano and N.M. Alves, “Antibacterial bioadhesive Layer-by-Layer coatings for orthopedic applications,” J. Mater. Chem. B, 4 (32), 5385-5393 (2016).
15. A.A. Abalymov, L. Van Der Meeren, M. Saveleva, E. Prikhozhdenko, K. Dewettinck, B. Parakhonskiy and A.G. Skirtach, “Cells-Grab-on Particles: A Novel Approach to Control Cell Focal Adhesion on Hybrid Thermally Annealed Hydrogels,” ACS Biomater. Sci. Eng., 6 (7), 3933-3944 (2020).
16. A.A. Abalymov, B. Parakhonskiy and A.G. Skirtach, “Polymer- and Hybrid-Based Biomaterials for Interstitial, Connective, Vascular, Nerve, Visceral and Musculoskeletal Tissue Engineering,” Polymers, 12 (3), 620 (2020).
17. T.A. Kolesnikova, D. Kohler, A.G. Skirtach and H. Möhwald, “Laser-Induced Cell Detachment, Patterning, and Regrowth on Gold Nanoparticle Functionalized Surfaces,” ACS Nano, 6 (11), 9585-9595 (2012).
18. J. Bai, X. Zuo, X. Feng, Y. Sun, Q. Ge, X. Wang and C. Gao, “Dynamic Titania Nanotube Surface Achieves UV-Triggered Charge Reversal and Enhances Cell Differentiation,” ACS Appl. Mater. Interfaces, 11 (40), 36939-36948 (2019).
19. R. Xing, T. Jiao, K. Ma, G. Ma, H. Möhwald and X. Yan, “Regulating Cell Apoptosis on Layer-by-Layer Assembled Multilayers of Photosensitizer-Coupled Polypeptides and Gold Nanoparticles,” Sci. Rep., 6, 26506 (2016).
20. P-G. De Gennes, “Soft Matter” (Nobel Lecture),” Angew. Chem. Int. Ed. Engl., 31 (7), 842-845 (1992).
21. E.V. Skorb and H. Möhwald, 25th Anniversary Article: “Dynamic Interfaces for Responsive Encapsulation Systems,” Adv. Mater., 25 (36), 5029-5043 (2013).
22. J. Li, W. Gao, R. Dong, A. Pei, S. Sattayasamitsathit and J. Wang, “Nanomotor lithography,” Nat. Commun., 5, 5026 (2014).
23. M. Guix, A.K. Meyer, B. Koch and O.G. Schmidt, “Carbonate-based Janus micromotors moving in ultra-light acidic environment generated by HeLa cells in situ,” Sci. Rep., 6, 21701 (2016).
24. R. Dong, J. Li, I. Rozen, B. Ezhilan, T. Xu, C. Christianson, W. Gao, D. Saintillan, B. Ren and J. Wang, “Vapor-Driven Propulsion of Catalytic Micromotors,” Sci. Rep., 5, 13226 (2015).
25. W. Tong, X. Song and C. Gao, “Layer-by-layer assembly of microcapsules and their biomedical applications,” Chem. Soc. Rev., 41 (18), 6103-6124 (2012).
26. V. Vergaro, F. Scarlino, C. Bellomo, R. Rinaldi, D. Vergara, M. Maffia, F. Baldassarre, G. Giannelli, X. Zhang, Y.M. Lvov and S. Leporatti, “Drug-loaded polyelectrolyte microcapsules for sustained targeting of cancer cells,” Adv. Drug Deliv. Rev., 63 (9), 847-864 (2011).
27. T. Zhao, L. Chen, P. Wang, B. Li, R. Lin, A. Abdulkareem Al-Khalaf, W.N. Hozzein, F. Zhang, X. Li, and D. Zhao, “Surface-kinetics mediated mesoporous multipods for enhanced bacterial adhesion and inhibition,” Nat. Commun., 10 (1), 4387 (2019).
28. X.Y. Ling, I.Y. Phang, C. Acikgoz, M.D. Yilmaz, M.A. Hempenius, G.J. Vancso and J. Huskens, “Janus Particles with Controllable Patchiness and Their Chemical Functionalization and Supramolecular Assembly,” Angew. Chem. Int. Ed., 48 (41), 7677-7682 (2009).
29. A.B. Pawar and I. Kretzschmar, “Fabrication, Assembly, and Application of Patchy Particles,” Macromol. Rapid Commun., 31 (2), 150-168 (2010).
30. M. Yoshida, K-H. Roh, S. Mandal, S. Bhaskar, D. Lim, H. Nandivada, X. Deng and J. Lahann, “Structurally Controlled Bio-hybrid Materials Based on Unidirectional Association of Anisotropic Microparticles with Human Endothelial Cells,” Adv. Mater., 21 (48), 4920-4925 (2009).
31. R.T.Chen, B.W. Muir, G.K. Such, A. Postma, K.M. McLean and F. Caruso, “Fabrication of asymmetric “Janus” particles via plasma polymerization,” Chem. Commun., 46 (28), 5121-5123 (2010).
32. V.N. Paunov and O.J. Cayre, “Supraparticles and “Janus” Particles Fabricated by Replication of Particle Monolayers at Liquid Surfaces Using a Gel Trapping Technique,” Adv. Mater., 16 (9-10), 788-791 (2004).
33. Z. Li, D. Lee, M.F. Rubner and R.E. Cohen, “Layer-by-Layer Assembled Janus Microcapsules,” Macromolecules, 38 (19), 7876-7879 (2005).
34. J-Q. Cui and I. Kretzschmar, “Surface-Anisotropic Polystyrene Spheres by Electroless Deposition,” Langmuir, 22 (20), 8281-8284 (2006).
35. D. Kohler, N. Madaboosi, M. Delcea, S. Schmidt, B.G. De Geest, D.V. Volodkin, H. Möhwald and A.G. Skirtach, “Patchiness of embedded particles and film stiffness control through concentration of gold nanoparticles,” Adv. Mater., 24 (8), 1095-1100 (2012).
36. W. Xu, A. Rudov, A. Oppermann, S. Wypysek, M. Kather, R. Schroeder, W. Richtering, I.I. Potemkin, D. Wöll, and A. Pich, “Synthesis of Polyampholyte Janus-like Microgels by Coacervation of Reactive Precursors in Precipitation Polymerization,” Angew. Chem. Int. Ed., 59 (3), 1248-1255 (2020).
37. S.P.R. Kobaku, C.S. Snyder, R.G. Karunakaran, G. Kwon, P. Wong, A. Tuteja and G. Mehta, “Wettability Engendered Templated Self-Assembly (WETS) for the Fabrication of Biocompatible, Polymer–Polyelectrolyte Janus Particles,” ACS Macro Lett., 8 (11), 1491-1497 (2019).
38. Y. Ji, X. Lin, D. Wang, C. Zhou, Y. Wu and Q. He, “Continuously Variable Regulation of the Speed of Bubble-Propelled Janus Microcapsule Motors Based on Salt-Responsive Polyelectrolyte Brushes,” Chem. Asian J., 14 (14), 2450-2455 (2019).
39. J. Shao, M. Abdelghani, G. Shen, S. Cao, D.S. Williams and J.C.M. van Hest, “Erythrocyte Membrane Modified Janus Polymeric Motors for Thrombus Therapy,” ACS Nano, 12 (5), 4877-4885 (2018).
40. Y. Wu, X. Lin, Z. Wu, H. Möhwald and Q. He, “Self-Propelled Polymer Multilayer Janus Capsules for Effective Drug Delivery and Light-Triggered Release,” ACS Appl. Mater. Interfaces, 6 (13), 10476-10481 (2014).
41. Y. Wu, T. Si, X. Lin and Q. He, “Near infrared-modulated propulsion of catalytic Janus polymer multilayer capsule motors,” Chem. Commun., 51 (3), 511-514 (2015).
42. Y. Yoshizumi and H. Suzuki, “Self-Propelled Metal–Polymer Hybrid Micromachines with Bending and Rotational Motions,” ACS Appl. Mater. Interfaces, 9 (25), 21355-21361 (2017).
43. C. Marschelke, I. Raguzin, A. Matura, A. Fery and A. Synytska, “Controlled and tunable design of polymer interface for immobilization of enzymes: Does curvature matter?,” Soft Matter, 13 (5), 1074-1084 (2017).
44. Z. Lin, Z. Wu, X. Lin and Q. He, “Catalytic Polymer Multilayer Shell Motors for Separation of Organics,” Chem. Eur. J., 22 (5), 1587-1591 (2016).
45. N. Hu, B. Zhang, M. Gai, C. Zheng, J. Frueh and Q. He, “Forecastable and Guidable Bubble-Propelled Microplate Motors for Cell Transport, Macromol. Rapid Commun., 38 (11), 1600795 (2017).
46. J.B. Gilbert, J.S. O’Brien, H.S. Suresh, R.E. Cohen and M.F. Rubner, “Orientation-Specific Attachment of Polymeric Microtubes on Cell Surfaces,” Adv. Mater., 25, 5948-5952 (2013).
47. W. He, J. Frueh, Z. Wu and Q. He, “Leucocyte Membrane-Coated Janus Microcapsules for Enhanced Photothermal Cancer Treatment,” Langmuir, 32 (15), 3637-3644 (2016).
48. W. He, J. Frueh, Z. Wu and Q. He, “How Leucocyte Cell Membrane Modified Janus Microcapsules are Phagocytosed by Cancer Cells,” ACS Appl. Mater. Interfaces, 8 (7), 4407-4415 (2016).
49. W. He, J. Frueh, N. Hu, L. Liu, M. Gai and Q. He, “Guidable Thermophoretic Janus Micromotors Containing Gold Nanocolorifiers for Infrared Laser Assisted Tissue Welding,” Adv. Sci., 3 (12), 1600206 (2016).
50. D. Volodkin, A. Skirtach and H. Möhwald, “Bioapplications of light-sensitive polymer films and capsules assembled using the Layer-by-Layer technique,” Polym. Int., 61 (5), 673-679 (2012).
51. A.L. Rogach, N.A. Kotov, D.S. Koktysh, A.S. Susha and F. Caruso, “II–VI semiconductor nanocrystals in thin films and colloidal crystals,” Colloids Surf. A: Physicochem. Eng. Asp., 202 (2-3), 135-144 (2002).
About the author
Dr. Xavier Albort Ventura earned his Ph.D. in Chemical Engineering Industrial from the University of Barcelona Spain. He is a member of the Barcelona International Trade Fair and has organizing responsibility for the Environment Technical Meetings and at the Eurosurfas show. He is an electroplating consultant for more of 50 European companies from various countries, including Portugal, France, Spain, Italy and Germany. Dr. Ventura has been an active presenter at SUR/FIN, having given papers since 1987. He has also been an active participant at Interfinish, presenting papers and conducting workshops since 1984. He has published more than 200 papers in Spain, the United Kingdom, France, the United States and Brazil. Collaboration in technical books with written chapters on in compilation of different conference authors in a dozen books. He has won different national and international awards in the United States and was awarded the Sam Wyman Memorial Award at SUR/FIN 2004 in Chicago for the Best Light Metals paper, “Sealing Method for Anodizing Aluminum and Hard Anodizing and a Test to Determine the Seal Quality of Aluminum.”

Corresponding author
Xavier Albort Ventura, Professor and Consultant
University Politecnic of Barcelona Spain Research and Development
C. Carabela La Niña, 22-24 bis
Barcelona, Spain 08017
Phone: 34-93-205.49.36
Mobile: 34-60-1370304
E-mail: xavieralbort@ing-mediamb.com
RELATED CONTENT
-
Cyanide-Free Electroplating of Cu-Sn Alloys
This paper is a peer-reviewed and edited version of a presentation delivered at NASF SUR/FIN 2012 in Las Vegas, Nev., on June 13, 2012.
-
PFAS and the Surface Finishing Industry
NASF and its member companies have a long history of environmental stewardship, especially when it comes to PFAS.
-
Plastics and Plating on Plastics [1944]
This republished 1944 AES convention paper presents an historic perspective of the early days of plastics in surface finishing - using them and plating on them, in the waning years of World War II. The discussion reviews the uses of plastics in plating equipment and processing at that time, as well as the coating of the plastics themselves, with accompanying application photos. You will note that today’s conventional plating-on-plastics processes lay far in the future. Surprisingly, CVD processes are discussed.


















