Notes From the Field
User experience with an organically stabilized electroless nickel process
#surfin #pollution control
Electroless nickel has a unique range of properties, including consistency of deposit thickness, excellent corrosion protection, high and controllable hardness and wear resistance, controlled magnetic properties and others. EN processes form a deposit by the chemical reduction of nickel, which, depending on the reducing agent used, gives either a nickel-phosphorus or nickel-boron alloy.
This reaction must be managed to ensure that the reduction only takes place on the work and not on the tanks and equipment used for plating. To ensure this, EN baths require additives commonly known as stabilizers. Most stabilizers also give improved brightness to the EN nickel deposit.
Featured Content
Lead and cadmium have been the industry standards to provide both brightness and stability for the full range of electroless nickel processes over the last 30–40 years, are very well understood and result in extremely reliable baths. Use of these metals, however, is increasingly controlled, resulting in a search for replacement stabilizers. Although the main directives (RoHS, ELV and WEEE) allow limited use of lead and cadmium, these limits have severely curtailed lead use and effectively prohibited use of cadmium. One response to this is development of baths that are purely lead-stabilized; however, this gives semi-bright deposits and—depending on the size of additions made—may result in lead content that exceeds RoHS limits at some point in the bath life.
Another factor is that legislation on environmental issues will only become more intense. One effect of this is that EN users are demanding complete removal of lead from EN plating solutions. This has resulted in many new EN processes formulated to be lead- and cadmium-free. It is MacDermid Enthone’s experience that cadmium- and lead-free processes now make up more than 30% of the EN in Europe and the Americas.
Search for Replacements
The original cadmium- and lead-free processes often used bismuth—one of the original metallic stabilizers used in electroless nickel—as the main stabilizer. Over the next 30 years bismuth was replaced with more reliable, better-performing lead and cadmium. Reverting to bismuth stabilizing technology is therefore in some ways a step backwards. Use of bismuth can also be related to some recent issues with EN processes such as reduced shelf life, poor activation of copper substrates, and high stress in some plating solutions, so the move back to it as a main stabilizer has not been an unmitigated success.
Bismuth is one of many materials appearing on various watch lists, but there are no specific health and safety or environmental issues with its use in EN processes in the U.S. or Europe. However, our company made the decision to research EN processes containing no metal stabilizers, partly to make allowance for future legislation and also due to an element of dissatisfaction with existing cadmium- and lead-free processes.
Many products are now available as non-metallically stabilized chemistry, but this article will focus on our company’s NiKlad ELV 835, an EN bath containing 4–7% phosphorus. This phosphorus concentration results in a deposit with an excellent balance between hardness and corrosion resistance. Results reported here are from actual customers, mainly job shop platers, but results also include some in-house plating operations, where demands on the plating solution are often lower.
Bath Operation
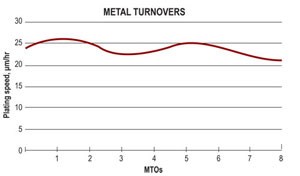
Organically stabilized EN solution has bath life comparable to other EN technologies. In the laboratory, users achieve eight metal turnovers (MTOs); at customer facilities with drag-out, life can be extended to 10 or more MTOs.
The bath can operate in both polypropylene and stainless steel tanks. For stainless tanks, this has included both nitric acid passivated and anodically protected installations. Stainless steel tanks are normally cleaned and passivated when a bath is dumped, which is also the case with organically stabilized bath. There’s no perceived advantage or disadvantage in moving away from metallically stabilized chemistry, although organic stabilizers are not plated out by the anodic protection equipment, making the bath more consistent.
With organically stabilized EN bath, the same chemistry is suitable for both polypropylene and stainless steel operations. Used in polypropylene tanks, the new chemistry gives slightly different performance than conventional . Anecdotal evidence suggests it is more stable than conventional systems, especially when used in old, scratched and damaged polypropylene tanks. One customer found that the tank plated up daily with existing ELV technology; with the new bath it can be used for three days before cleaning.
Organically stabilized baths have a high plating rate, which lasts throughout the bath life. Conventional solutions tend to slow as they age, partly due to build-up of contaminants such as orthophosphate, sulfate, ammonia or sodium, but also due to the need with metallic stabilizers for the metal concentration to slowly rise as the bath ages.
The high speed of organically stabilized baths can have some unplanned side effects. One customer using the bath found that when plating at more than 1mil/hr, operators could not keep pace with the solution. They slowed the bath down to 0.8mils/hr by reducing bath temperature. This also had the effect of reducing energy costs, and they were still obtaining a higher plating rate than their previous solution.
One of the major advantages seen by platers operating the organically stabilized baths is the lack of sensitivity to additions compared with metallically stabilized solutions. One plater operating the bath at 3g/L of nickel allowed nickel concentration to fall to 1.2g/L nickel. Rather than making several small additions to bring the bath back to strength, they added one large addition. In a metallically stabilized solution, this would have stopped plating or at best given skip plating. The organically stabilized bath continued to plate as normal.
This is an advantage when using the bath as a low-metal operation, because it means that auto-dosing is not needed to operate at nickel concentration below 6g/L. The mechanism that allows this is not clear but must be related to the strength of the stabilizer and the fact that normal transport mechanisms are not as critical as with the metallic atoms.
Ability to tolerate large additions is also important for platers operating with low (<0.1ft2/gal) bath loadings. The normal reduction in plating rate due to constant low loading is not an issue with organically stabilized chemistry.
Conversely, using much higher bath loadings (>1.5ft2/gal) is also acceptable. Despite this wider window of operation and control, organically stabilized EN baths, like all plating baths, perform best when operated within tight parameters, ideally between 90–105%.
Substrates
Some of the new RoHS-compliant EN chemistries have suffered from poor activation when plating some steel substrates (for example, materials with a high lead content), copper and brass (especially when parts are activated in plating solution) and aluminum. This is believed to be due to higher concentration of non-lead stabilizers in the solutions. Use of organically stabilized solutions has resulted in fewer issues. An advantage of the organically stabilized bath is its life when plating aluminum. A conventional EN solution used without a strike bath normally will not plate more than four MTOs on aluminum due to the increased risk of adhesion failure. One customer using the new chemistry to plate mainly on aluminum found they could still continue to five MTOs without a strike, even though the part is machined (not cast) and is hot solder dipped after plating—a tough adhesion test. The reasons for this are not certain, but there may be two factors at work: 1)A possible increased tolerance to metallic contamination; and 2)Low stress in the bath as it ages.
Additive Stability
To ensure solubility of the lead and bismuth used as stabilizers, it’s common for metal-stabilized EN baths to contain strong complexors, normally EDTA or derivatives of EDTA. The result is that spent baths are more difficult to waste treat than solutions not containing these compounds. Because new solutions use no metals other than nickel, these materials are not used in the bath.
Even using very strong complexors, the shelf life of many ELV-compliant chemistries is shorter than conventional lead-stabilized systems, as the metals can slowly precipitate. This can cause inconsistent performance and a short shelf life. Bismuth is even worse; it can be difficult to prevent its precipitation without moving to a four-component system.
Deposit Composition
As-plated deposits from organically stabilized baths look slightly different than deposits from conventional and metallically stabilized systems—they are “whiter” and less yellow. This is due to lack of metallic stabilizer being co-deposited, which can be as high as 0.15% if cadmium and lead are used and 0.1% for bismuth.
With organically stabilized EN, the only materials co-deposited with the phosphorus and nickel are carbon and sulfur with the low- and medium-phosphorus systems, and carbon with the high-phosphorus bath. In a comparison of 4–6% phosphorus deposits plated using a lead-cadmium and an organically stabilized bath, the conventional chemistry co-deposited <200 ppm carbon and 173 ppm sulfur when new and <400 ppm carbon and 414 ppm sulfur after six MTOs. A similar deposit from an organically stabilized bath co-deposited <400 ppm carbon and <50 ppm sulfur when new; after six MTOs carbon and sulfur contents in the deposit were <200 ppm and 561 ppm, respectively.
The organically stabilized bath also shows a low/medium level of phosphorus throughout its life, with the level falling slightly as the bath ages.
Deposit Properties
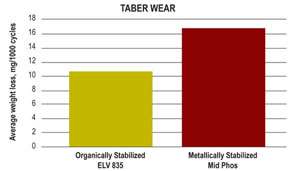
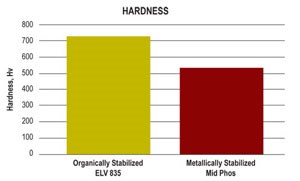
Deposits from organically stabilized EN baths are harder than those from a conventional medium-phosphorus bath. This is more related to phosphorus content than the stabilizers used in the bath.
Wear resistance measured using a Taber wear test is reduced in line with the extra hardness seen, while corrosion resistance of the deposit tested using electrochemical means was as good as conventional systems with slightly higher phosphorus content. Neutral salt-spray testing was then carried out to confirm results. Performance when the bath was new was very good, but it decreased slightly as the solution aged. This is seen as a result of increased porosity, and in practice, user parts tested side by side with metallically stabilized materials were found to give the same hours salt-spray resistance.
Deposits from organically stabilized baths show no signs of high tensile stress throughout bath life. The bath starts off slightly tensile and slowly becomes less, sometimes even crossing over to the compressive side. This is a function of managing phosphorus content of the bath and maintaining the speed of the solution throughout its life. It is also a factor in assessing the ability of the bath to continue to plate quality deposits as it ages. In conventional EN systems, the growth of tensile stress as the bath ages has been a limiting factor in bath life.
Although it has little effect on performance of the deposit, brightness is still a major contributor to perception of quality—it has happened, but we have rarely come across an application where the customer wants a duller deposit. The organically stabilized chemistry can produce bright deposits, but it does not yet match metallically stabilized processes in all installations. Users have said that the gloss and brightness of the deposit do not change as much as conventional systems and remain quite consistent for the life of the solution
The authors thank Carl Steinecker, Nicole Micyus, John Szczypka and Marko Duffy for their assistance in preparing this article, which is based on a presentation given at SUR/FIN 2008.
RELATED CONTENT
-
Cyanide Destruction: A New Look at an Age-Old Problem
Cyanide in mining and industrial wastewaters has been around from the beginning, including electroplating processes. This presentation reviews a number of current processes, and in particular, offers new technologies for improvement in cyanide destruction by the most common process, using sodium hypochlorite.
-
Hybrid Sol-Gel Coatings in Surface Engineering
A look at the use of modified sol-gel polymer films and hybrid system coatings, as well as the methodologies for evaluating the mechanical properties of the coatings.
-
A Pulse/Pulse Reverse Electrolytic Approach to Electropolishing and Through-Mask Electroetching
Research at the authors’ laboratories has focused on pulse/pulse reverse electrolysis on cathodic processes, such as hard chromium plating from non-hexavalent chemistries. This papers describes studies into pulse/pulse reverse electrolysis as applied to electrochemical metal removal processes, such as electropolishing and electroetching.