Silver Keeps Pace
Electroplating chemistries evolve to meet electronics industry needs
#electronics #pollutioncontrol
Silver plating is an old process, but one that has steadily evolved in response to changing customer requirements. Many of the changes to silver plating baths have come in response to the needs of the electronics industry. This continues to be the case even today, when environmental requirements and new potential applications in the photovoltaic (solar) market are driving the changes.
From an environmental standpoint, silver plating—and precious metal plating in general—has not been considered a problem. Platers recovered the expensive metal from spent solutions and dragouts. Any cyanide-containing effluents were easily treated.
Featured Content
But developments in legislation and current perceptions regarding the environment are now forcing changes to the chemicals used in precious metal plating processes. New silver plating formulations include low-cyanide and non-cyanide systems that eliminate potentially hazardous chemicals from both the plating bath and from the deposited metals.
Following is a look at past and present silver plating chemistries and applications as well as a discussion of the forces and new applications that are driving current formulation changes.
A Brief History
Silver plating baths have been around for more than 100 years. Originally produced from alkaline cyanide electrolytes by dissolving silver cyanide in a solution of sodium cyanide, silver plating formulations soon switched to “cleaner” baths based on silver potassium cyanide. Known as the double salt, this chemistry eliminated the use of “dusty” silver cyanide, simplified bath replenishment and eliminated the need to filter residues.
Because silver cyanide plating baths tended to produce coatings with poor adhesion, they were always used in conjunction with a strike bath. Silver strikes usually operated at ambient temperature and had a low silver content and high cyanide content. This formulation produced a very thin layer of adherent silver on the base material. Powdering was minimized by keeping plating time very short. Such high-cyanide (100–150 g/L KCN) silver baths used a variety of anode types, including silver sheet, dog bone and corrugated shapes, as different types of silver grain.
Originally used almost exclusively for decorative plating, silver use increased rapidly during the 1960s and 1970s as the price of gold rose and silver became a mainstay of the electronics plating industry. Silver cyanide-based baths coupled with semi-metallic (for decorative applications) or organic (decorative and electronic plating) brighteners became very popular due to their ease of operation, consistent quality and ease of effluent treatment. This is still true today, although formulators continued to develop these relatively simple silver baths throughout the 1970s in response to the electronics industry’s need to produce very high-speed plating solutions for semiconductor production.
Requirements from the semiconductor market for selectively silver plated lead frames also led to development of a new generation of silver processes originally claimed to be non-cyanide systems. These were actually low-cyanide systems that contained no free cyanide on makeup but were replenished by salt, resulting in a buildup of some free cyanide during the bath life.
Silver also began to see application as a lead-free alternative for solderable finishes on printed circuit boards (PCBs). Processes containing silver nitrate with an organic solderable coating are readily available in the market but are used almost exclusively in the PCB industry. There have also been attempts to develop alloy plating systems, such as silver/tin and silver/copper, but none of these formulations has been developed into a usable commercial manufacturing process.
As the electronics industry emerged fully during the 1970s, the need to plate silver at high speeds became very apparent. High-cyanide bright silver chemistries were used quite successfully for barrel plating applications.
However, these baths had limitations when used at high current densities on high-speed reel-to-reel lines and selective spot lines in particular. Selective plating of silver spots onto lead frames required vastly different plating conditions and parameters, resulting in development of a new range of silver plating solutions. These chemistries still used the double salt silver potassium cyanide, but were very different from conventional silver cyanide baths.
Low-Cyanide Baths
These low-cyanide silver plating processes have now been in use for more than 30 years. They contain no free cyanide, so they don’t dissolve silver anodes. They therefore use dimensionally stable anodes (DSAs) such as platinized titanium. They contain zero free cyanide on makeup and are replenished by addition silver potassium cyanide.
Generally, low-cyanide baths have a finite life and slowly build up cyanide over the course of their service life. They often contain approximately 20g/L of free cyanide at the end of their life; however, it is carbonate build up the denotes the end of life. The conductivity of the process is supported by either phosphate or nitrate chemistry to replace the cyanide. Because these baths are capable of high production throughputs, they must be replaced regularly.
These baths have many advantages from both production and n environmental/worker safety standpoints. They eliminate the need for silver strike to promote adhesion, and they plate at extremely high rates that support high production levels. They also provide a safer working environment with reduced heath and safety costs and a reduced environmental impact from low cyanide and elimination of other potential pollutants.
Low-cyanide, high-speed silver plating baths are usually operated with metal content of about 65 g/L of silver, pH of 8.5 and bath temperature of 65°C. Phosphate-based silver baths can deposit up to 0.1 μm/sec of silver, while nitrate-based baths can deposit up to 0.5 μm/sec of metal. More complete operating parameters and permissible operating ranges are shown in Table 1.
Cyanide-Free Silver
The latest silver plating chemistries eliminate cyanide completely, and therefore offer significant environmental and safety benefits even in comparison with low-cyanide bath formulations. These baths are made by combining a complex of silver methane sulfonate (MSA) with a new, patented complex to replace the cyanide. The bath is straightforward to make up, and uses a wetting agent and brighteners similar to those used in traditional cyanide silver baths. It will also readily dissolve silver anodes, avoiding some of the cost issues of other non-cyanide baths that require additions of silver salts.
Like low-cyanide chemistries, cyanide-free baths promote worker health and safety, have reduced environmental impact and require no strike to promote adhesion of the main silver deposit. They also contain no carbonates, which increases bath life, and are fully analyzable, which makes them easier to operate. Table 2 lists the usual operating parameters and permissible working ranges of cyanide-free silver plating solutions.
Clearly, environmental and worker safety regulations are not going to be relaxed for potentially hazardous processes such as electroplating—if anything, they will continue being tightened. Although they are currently perceived as being at a cost disadvantage relative to tradition processes, low- and non-cyanide silver plating processes will become more prevalent in the coming years as more platers become aware of the “real” costs of using hazardous materials.
Bright New Application
Recently there has been a great deal of discussion regarding development of clean, renewable energy sources to replace coal, natural gas and other carbon-based fuels. One such technology uses silver as a conductor to transform solar energy into electricity via a silicon wafer.
Over the past few years the market for solar-powered energy has grown substantially, and the need for solar photovoltaic (PV) cells has grown with it. World solar photovoltaic installations for electricity production reached a record high of 1,744 MW in 2006, representing growth of 19% over the previous year. Germany’s grid-connect PV market grew 16% to 960 MW, and now accounts for 55% of the world market. While Japan’s market size barely grew last year, Spain and the United States were both strong performers: The Spanish market was up more than 200% in 2006 while the US market grew 33%.
On the supply side, world solar cell production reached a consolidated figure of 2,204 MW in 2006, up from 1,656 MW a year earlier. Japanese producers lost ground, dropping from 46% to 39% share, to the benefit of Chinese cell manufacturers. Polysilicon production rose 16% in 2006, which, when combined with aggressive PV industry procurement, allowed a marginally higher market growth rate than projected twelve months ago. Nonetheless, polysilicon supply issues will still constrain cell production in 2007.
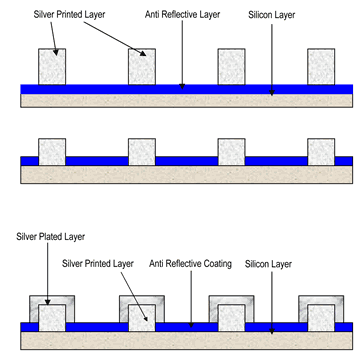
Traditionally, the silver conductor in PV cells is applied by screen printing a silver paste onto the silicon wafer, which has an anti reflective coating applied. On firing, the silver paste burns through the anti-reflective coating, bonding to the silicon substrate and creating the circuit. Estimates are that fabrication of PV cells will consume 270 metric tons of silver ink this year—one third in Europe, one third in Asia (mostly Japan) and one third in the rest of the world, mostly the U.S.
Engineers designing PV cells and processes would like a silver layer that is as continuous, consistent and conductive as possible. The screen printed, fired films have some difficulties with continuity, opening the door to possible use of silver electroplating in this application.
The technique involves use of a narrow strip of screen-printed silver. After firing, this silver layer serves as the seed layer for a layer of electroplated silver which can be applied very easily to this screen-printed coating. The process plates a continuous, pure silver layer 10 μm thick that results in more efficient transfer of collected solar energy to electricity, making application of this technology very interesting to PV design engineers.
One of the technical issues with use of silver electroplating in this application is development of handling systems for the thin silicon wafers to achieve realistic plating production times. Also, new silver inks will be required to achieve a fine dense, thin, narrow seed layer for the thicker plating layer.
RELATED CONTENT
-
Electroplating, Electrochemistry and Electronics - The 15th William Blum Lecture - Part 3
This article is the third of four parts of a re-publication of the 15th William Blum Lecture, presented at the 61st AES Annual Convention in Chicago, Illinois, on June 17, 1974. Dr. George Dubpernell reviews the history and extent of commercial plating, then delves into the electrochemical science, including potentials, overvoltage and connections to electronics.
-
Thin Multilayer Palladium Coatings for Semiconductor Packaging Applications Part I: Solderability
The 1996 AES Gold Medal Award was given to I.V. Kadija and his co-workers for the Best Paper appearing in Plating and Surface Finishing in 1995. Their work dealt with the use of palladium in electronics finishing in the 1990s. This paper covered the evaluation of such finishes for solderability.
-
RoHS and ELV Compliant Electroless Nickel
Over the past few years, a number of new environmental directives have come out of Europe and Asia encompassing mainly the automotive and electronics industries.